Abstract
Introduction
Colchicine is currently approved for the treatment of gout and familial Mediterranean fever, among other conditions. Clarithromycin, a strong inhibitor of CYP3A4 and P-glycoprotein, dramatically increases colchicine’s half-life, augmenting the risk of a life-threatening adverse reaction when used inadvertently with colchicine.
Objectives
The aim of this study was to examine the evidence and clinical implications of concomitant use of colchicine and clarithromycin.
Methods
Case reports of colchicine–clarithromycin co-administration were searched using the FDA’s Adverse Event Reporting System (FAERS) database. PubMed, EMBASE, and Web of Science electronic databases were also searched from January 2005 through November 2019 for articles reporting colchicine–clarithromycin concomitant use. Individual reports were reviewed to identify consequences of coadministration, dose, days to onset of interaction, symptoms, evidence of renal disease, time to resolution of symptoms, and Drug Interaction Probability Scale (DIPS) rating.
Results
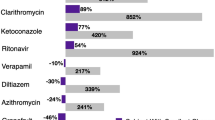
The FAERS search identified 58 reported cases, nearly 53% of which were from patients aged between 65 and 85 years. Of 30 reported deaths, 11 occurred in males, and 19 in females. Other frequent complications reported in FAERS included diarrhea (31%), pancytopenia (22%), bone marrow failure (14%), and vomiting (14%). From published literature, we identified 20 case reports of concomitant exposure, 19 of which were rated ‘probable’ and one ‘possible’ according to DIPS rating. Of these cases, four ‘probable’ patients expired. The documented onset of colchicine toxicity occurred within 5 days of starting clarithromycin, and death within 2 weeks of concomitant exposure.
Conclusion
Clinical manifestations of colchicine–clarithromycin interaction may resemble other systemic diseases and may be life threatening. Understanding this clinically meaningful interaction can help clinicians avoid unsafe medication combinations.
Similar content being viewed by others
References
Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res Ther [Internet]. BioMed Central; 2006;8:S1.
Ozen S, Demirkaya E, Erer B, et al. EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis. 2016;75(4):644–51. https://doi.org/10.1136/annrheumdis-2015-208690.
Leung YY, Yao Hui LL, Kraus VB. Colchicine--Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum [Internet]. NIH Public Access; 2015;45:341–50.
Bhattacharyya B, Panda D, Gupta S, Banerjee M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev. 2008;28:155–83.
Sackett DL, Varma JK. Molecular mechanism of colchicine action: induced local unfolding of β-tubulin. Biochemistry. 1993;32:13560–5.
Caviston JP, Holzbaur ELF. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–7.
Ben-Chetrit E, Fischel R, Hinz B, Levy M. The effects of colchicine and hydroxychloroquine on the cyclo-oxygenases COX-1 and COX-2. Rheumatol Int. 2005;25:332–5.
Nuki G. Colchicine: Its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. 2008;10:218–27.
Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. 1995;96:994–1002.
Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, et al. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol. 2010;48:407–14.
Todd BA, Billups SJ, Delate T, Canty KE, Kauffman AB, Rawlings JEWT. Assessment of the association between colchicine therapy and serious adverse events. Pharmacotherapy. 2012;32:974–80.
Villamañán E, Larrubia Y, Ruano M. Colchicine: what’s up, doc? Med Clin (Barc). 2012;139:295–9.
Terkeltaub RA, Furst DE, Digiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63:2226–37.
Zuckerman JM, Qamar F, Bono BR. Review of macrolides (azithromycin, clarithromycin), ketolids (telithromycin) and glycylcyclines (tigecycline). Med Clin North Am. 2011;95:761–91.
Davidson RJ. In vitro activity and pharmacodynamic/pharmacokinetic parameters of clarithromycin and azithromycin: why they matter in the treatment of respiratory tract infections. Infect Drug Resist. 2019;12:585–96. https://doi.org/10.2147/IDR.S187226.
Hung IFN, Wu AKL, Cheng VCC, Tang BSF, To KW, Yeung CK, et al. Fatal interaction between clarithromycin and colchicine in patients with renal insufficiency: a retrospective Study. Clin Infect Dis. 2005;41:291–300.
Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction cases. Ann Pharmacother. 2007;41:674–80.
Hakozaki Y, Mitani K, Okada C, Terada H, Kobari S. Effective Treatment of intestinal Behçet’s disease with long-term, low-dose clarithromycin. Case Rep Gastroenterol. 2013;7:122–6.
Amanova A, Kendi Celebi Z, Bakar F, Caglayan MG, Keven K. Colchicine levels in chronic kidney diseases and kidney transplant recipients using tacrolimus. Clin Transpl. 2014;28:1177–83.
Cohen O, Locketz G, Hershko AY, Gorshtein A, Levy Y. Colchicine-clarithromycin-induced rhabdomyolysis in familial mediterranean fever patients under treatment for Helicobacter pylori. Rheumatol Int. 2015;35:1937–41.
Haj Yahia S, Ben Zvi I, Livneh A. Colchicine intoxication in familial Mediterranean fever patients using clarithromycin for the treatment of Helicobacter pylori: a series of six patients. Rheumatol Int. 2018;38:141–7.
Little A, Tung D, Truong C, Lapinsky S, Burry L. Colchicine overdose with coingestion of nonsteroidal antiinflammatory drugs. CJEM. 2014;16:252–6.
Baud FJ, Sabouraud A, Vicaut E, Taboulet P, Lang J, Bismuth C, et al. Brief report: treatment of severe colchicine overdose with colchicine-specific Fab fragments. N Engl J Med. 1995;332:642–5.
Food and Drug Administration. FDA AEs reporting system (FAERS) public dashboard. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm. Accessed Nov 2019.
Francis L, Bonilla E, Soforo E, Neupane H, Nakhla H, Fuller C, et al. Fatal toxic myopathy attributed to propofol, methylprednisolone, and cyclosporine after prior exposure to colchicine and simvastatin. Clin Rheumatol. 2007;27:129–31.
Cocco G, Chu DCC, Pandolfi S. Colchicine in clinical medicine. A guide for internists. Eur J Intern Med. 2010;21:503–8.
Caraco Y, Putterman C, Rahamimov R, Ben-Chetrit E. Acute colchicine intoxication–possible role of erythromycin administration. J Rheumatol. 1992;19:494–6.
Hansten PD, Horn JR. The Top 100 DRUG Interactions. A guide to patient management. Freeland: H&H Publications; 2019.
Dogukan A, Oymak FS, Taskapan H, Güven M, Tokgoz BUC. Acute fatal colchicine intoxication in a patient on continuous ambulatory peritoneal dialysis (CAPD). Possible role of clarithromycin administration. Clin Nephrol. 2001;55:181–2.
Rollot F, Pajot O, Chauvelot-Moachon L, Nazal EM, Kélaïdi C, Blanche P. Acute colchicine intoxication during clarithromycin administration. Ann Pharmacother. 2004;38:2074–7.
Cheng VCC, Ho PL, Yuen KY. Two probable cases of serious drug interaction between clarithromycin and colchicine. South Med J. 2005;98:811–3.
Akdag I, Ersoy A, Kahvecioglu S, Gullulu M DK. Acute colchicine intoxication during clarithromycin administration in patients with chronic renal failure. J Nephrol. 2006;19:515–7.
van der Velden W, Huussen J, Ter Laak H, de Sévaux R. Colchicine-induced neuromyopathy in a patient with chronic renal failure: the role of clarithromycin. Neth J Med. 2008;66:204–6.
McKinnell J, Tayek JA. Short term treatment with clarithromycin resulting in colchicine-induced rhabdomyolysis. J Clin Rheumatol. 2009;15:303–5.
Izquierdo Pajuelo MJ, Jiménez Delgado JD, Rangel Mayoral JF, Liso Rubio FJ. Interacción mortal entre colchicina y claritromicina. Farm Hosp. 2010;34:309–10.
Kim J-B, Kim S, Lee T, Lee YS, Cho YS, Moon H-B, et al. Colchicine-induced rhabdomyolysis caused by interaction with clarithromycin in a patient with Behcet disease. J Clin Rheumatol. 2013;19:108–9.
Çelebi ZK, Akturk S, Oktay EI, Duman N, Keven K. Colchicine-induced rhabdomyolysis following a concomitant use of clarithromycin in a haemodialysis patient with familial Mediterranean fever. Clin Kidney J. 2013;6:665–6.
Olmos-Martínez JM, Molina H, Salas C, Olmos JM, Hernández JL. Acute clchicine-induced neuromyopathy in a patient treated with atorvastatin and clarithromycin. Eur J Case Rep Intern Med. 2019;6(3):001066. https://doi.org/10.12890/2019_001066.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was supported by grant R01HS025984 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Conflict of interest
All authors have completed the ADIS uniform disclosure. There was no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval
None.
Data availability
Reports of colchicine–clarithromycin drug interactions in FAERS can be publicly accessed via: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard
Disclaimer
The authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Rights and permissions
About this article
Cite this article
Villa Zapata, L., Hansten, P.D., Horn, J.R. et al. Evidence of Clinically Meaningful Drug–Drug Interaction With Concomitant Use of Colchicine and Clarithromycin. Drug Saf 43, 661–668 (2020). https://doi.org/10.1007/s40264-020-00930-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-020-00930-7