Abstract
Introduction
Proton pump inhibitors (PPIs) have been implicated in the occurrence of moderate to severe myopathies in several case reports.
Aim
This study was performed to assess the reporting risk of muscular adverse drug reactions (ADRs) associated with PPIs in the Italian National Network of Pharmacovigilance database.
Methods
A disproportionality analysis (case/non-case) was performed using spontaneous reports collected in the database between July 1983 and May 2016. Reporting odds ratio (ROR) and 95% confidence intervals (CIs) were calculated as a measure of disproportionality. In a secondary and tertiary analysis, we explored the association of PPIs with muscular ADRs after taking into account the masking effect of statins. Moreover, the possibility of an interaction between PPIs and statins, leading to the occurrence of muscular ADRs, was also tested.
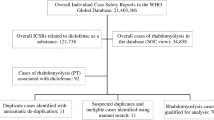
Results
The study was carried out on 274,108 reports. The ROR of muscular ADRs for PPIs, adjusted for age and gender, was 1.484 (95% CI 1.204–1.829; p < 0.001), whereas the ROR for rhabdomyolysis was 0.621 (95% CI 0.258–1.499). Similar results were obtained in the secondary analysis. The tertiary analysis, where PPIs were considered regardless of whether their role was suspected or concomitant, showed a potential disproportionate reporting for the combination PPIs–rhabdomyolysis (ROR 1.667, 95% CI 1.173–2.369; p < 0.01). The PPIs–statins combination was not associated with an enhanced ROR of muscular ADRs/rhabdomyolysis compared with statins alone.
Conclusions
This explorative study suggests that the class of PPIs could be involved in reports of muscular ADRs, rather than any other ADR, more frequently than any non-statin drug. Our results must be corroborated by further studies.

Similar content being viewed by others
References
Dalakas MC. Toxic and drug-induced myopathies. J Neurol Neurosurg Psychiatry. 2009;80(8):832–8.
Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40(3):567–72.
Alis R, Sanchis-Gomar F, Risso-Ballester J, et al. Inhibition of xanthine oxidase to prevent statin-induced myalgia and rhabdomiolysis. Atherosclerosis. 2015;239(1):38–42.
Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346(7):539–40.
Motola D, Vargiu A, Leone R, et al. Influence of regulatory measures on the rate of spontaneous adverse drug reaction reporting in Italy. Drug Saf. 2008;31(7):609–16.
McAdams M, Staffa J, Dal Pan G. Estimating the extent of reporting to FDA: a case study of statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2008;17(3):229–39.
Conforti A, Chiamulera C, Moretti U, et al. Musculoskeletal adverse drug reactions: a review of literature and data from ADR spontaneous reporting databases. Curr Drug Saf. 2007;2(1):47–63.
Bebarta VS, King JA, McDonough M. Proton pump inhibitor-induced rhabdomyolysis and hyponatremic delirium. Am J Emerg Med 2008;26(4):519 e1–2.
Tanaka K, Nakada TA, Abe R, et al. Omeprazole-associated rhabdomyolysis. Crit Care. 2014;18(4):462.
Troger U, Reiche I, Jepsen MS, et al. Esomeprazole-induced rhabdomyolysis in a patient with heart failure. Intensive Care Med. 2010;36(7):1278–9.
Di Grande A, Giustolisi V, Tabita V, et al. Hypokalemic rhabdomyolysis in a patient with a laparoscopic adjustable gastric banding. Clin Ter. 2008;159(3):169–72.
Garrote FJ, Lacambra C, del Ser T, et al. Subacute myopathy during omeprazole therapy. Lancet. 1992;340(8820):672.
Tuccori M, Giovannoni S, Giustini SE, et al. Acute severe myopathy following a single infusion of omeprazole. Ann Pharmacother. 2006;40(2):352–3.
Nozaki M, Suzuki T, Hirano M. Rhabdomyolysis associated with omeprazole. J Gastroenterol. 2004;39(1):86.
Grattagliano I, Portincasa P, Mastronardi M, et al. Esomeprazole-induced central fever with severe myalgia. Ann Pharmacother. 2005;39(4):757–60.
Visruthan NK, Boo PK, Kader A, et al. Omeprazole-induced myositis in a child receiving triple therapy for Helicobacter pylori infection. J Pediatr Gastroenterol Nutr. 2012;55(3):338–9.
Sivakumar K, Dalakas MC. Autoimmune syndrome induced by omeprazole. Lancet. 1994;344(8922):619–20.
Villa A, Martinoli E, Nucera G, et al. Proton pump inhibitor-induced hypomagnesemia. Italian J Med. 2014;8(s2):134.
Bilbao JM, Moddel G. Autophagic myopathy induced by omeprazole therapy. Can J Neurol Sci. 1998;25(4):339.
Colmenares EW, Pappas AL. Proton pump inhibitors: risk for myopathy? Ann Pharmacother 2016. doi:10.1177/1060028016665641.
Jeon DH, Kim Y, Kim MJ, et al. Rhabdomyolysis associated with single-dose intravenous esomeprazole administration: a case report. Med (Baltimore). 2016;95(29):e4313.
Wang AK, Sharma S, Kim P, et al. Hypomagnesemia in the intensive care unit: choosing your gastrointestinal prophylaxis, a case report and review of the literature. Indian J Crit Care Med. 2014;18(7):456–60.
Clark DW, Strandell J. Myopathy including polymyositis: a likely class adverse effect of proton pump inhibitors? Eur J Clin Pharmacol. 2006;62(6):473–9.
Ruiter R, Visser LE, van Herk-Sukel MP, et al. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia. 2012;55(1):51–62.
Colmers IN, Bowker SL, Tjosvold LA, et al. Insulin use and cancer risk in patients with type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Metab. 2012;38(6):485–506.
Bronsveld HK, ter Braak B, Karlstad O, et al. Treatment with insulin (analogues) and breast cancer risk in diabetics; a systematic review and meta-analysis of in vitro, animal and human evidence. Br Cancer Res BCR. 2015;17:100.
Grimaldi-Bensouda L, Cameron D, Marty M, et al. Risk of breast cancer by individual insulin use: an international multicenter study. Diabetes Care. 2014;37(1):134–43.
Elazzazy S, Eziada SS, Zaidan M. Rhabdomyolysis secondary to drug interaction between atorvastatin, omeprazole, and dexamethasone. Int Med Case Rep J. 2012;5:59–61.
Sipe BE, Jones RJ, Bokhart GH. Rhabdomyolysis causing AV blockade due to possible atorvastatin, esomeprazole, and clarithromycin interaction. Ann Pharmacother. 2003;37(6):808–11.
Kanth R, Shah MS, Flores RM. Statin-associated polymyositis following omeprazole treatment. Clin Med Res. 2013;11(2):91–5.
Marusic S, Lisicic A, Horvatic I, et al. Atorvastatin-related rhabdomyolysis and acute renal failure in a genetically predisposed patient with potential drug–drug interaction. Int J Clin Pharm. 2012;34(6):825–7.
Hylton AC, Ezekiel TO. Rhabdomyolysis in a patient receiving ranolazine and simvastatin. Am J Health Syst Pharm. 2010;67(21):1829–31.
Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109–17.
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ (Clin Res Ed). 2005;330(7503):1304–5.
Yue Z, Shi J, Jiang P, et al. Acute kidney injury during concomitant use of valacyclovir and loxoprofen: detecting drug–drug interactions in a spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2014;23(11):1154–9.
de Langen JJ, van Puijenbroek EP. HMG-CoA-reductase inhibitors and neuropathy: reports to the Netherlands Pharmacovigilance Centre. Neth J Med. 2006;64(9):334–8.
Cakir M, Samanci N, Balci N, et al. Musculoskeletal manifestations in patients with thyroid disease. Clin Endocrinol (Oxf). 2003;59(2):162–7.
Lindner LS, Emkey GR. Exertion-related rhabdomyolysis observed with hyperthyroidism. Am J Med. 2015;128(6):e7–8.
van Puijenbroek EP, Egberts AC, Heerdink ER, et al. Detecting drug–drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol. 2000;56(9–10):733–8.
Van Puijenbroek EP, Egberts AC, Meyboom RH, et al. Signalling possible drug–drug interactions in a spontaneous reporting system: delay of withdrawal bleeding during concomitant use of oral contraceptives and itraconazole. Br J Clin Pharmacol. 1999;47(6):689–93.
Egberts AC, Meyboom RH, van Puijenbroek EP. Use of measures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf. 2002;25(6):453–8.
Piccinni C, Gissi DB, Gabusi A, et al. Paraesthesia after local anaesthetics: an analysis of reports to the FDA Adverse Event Reporting System. Basic Clin Pharmacol Toxicol. 2015;117(1):52–6.
Hauben M, Reich L. Communication of findings in pharmacovigilance: use of the term “signal” and the need for precision in its use. Eur J Clin Pharmacol. 2005;61(5–6):479–80.
Hauben M, Hung EY. Revisiting the reported signal of acute pancreatitis with rasburicase: an object lesson in pharmacovigilance. Ther Adv Drug Saf. 2016;7(3):94–101.
Maignen F, Hauben M, Hung E, et al. Assessing the extent and impact of the masking effect of disproportionality analyses on two spontaneous reporting systems databases. Pharmacoepidemiol Drug Saf. 2014;23(2):195–207.
Wang HW, Hochberg AM, Pearson RK, et al. An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf. 2010;33(12):1117–33.
Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case–control study. BMC Nephrol. 2013;14:150.
Harper CR, Jacobson TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep. 2010;12(5):322–30.
Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother. 2001;35(9):1096–107.
Sathasivam S, Lecky B. Statin induced myopathy. BMJ. 2008;337:a2286.
Juhlin K, Ye X, Star K, et al. Outlier removal to uncover patterns in adverse drug reaction surveillance—a simple unmasking strategy. Pharmacoepidemiol Drug Saf. 2013;22(10):1119–29.
van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Coulter D. Does omeprazole cause polymyositis? Proceedings of the Royal Australasian College of Physicians, Annual Clinical and Scientific meeting, 1996:81 (abstract).
Coulter DM. Signal generation in the New Zealand Intensive Medicines Monitoring Programme: a combined clinical and statistical approach. Drug Saf. 2002;25(6):433–9.
Gould AL. Practical pharmacovigilance analysis strategies. Pharmacoepidemiol Drug Saf. 2003;12(7):559–74.
Pariente A, Avillach P, Salvo F, et al. Effect of competition bias in safety signal generation: analysis of a research database of spontaneous reports in France. Drug Saf. 2012;35(10):855–64.
Hauben M, Hochberg A. The importance of reporting negative findings in data mining. Pharm Med. 2008;22(4):215–9.
Salvo F, Leborgne F, Thiessard F, et al. A potential event-competition bias in safety signal detection: results from a spontaneous reporting research database in France. Drug Saf. 2013;36(7):565–72.
Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013;6(4):443–51.
Wolfe-Wylie M, Mouzaki M, Sochett E, et al. chronic high dose proton-pump inhibitors as a cause of hypophosphatemic rickets. Endocr Rev 2016. doi:10.1210/endo-meetings.2016.BCHVD.16.FRI-348.
Su SS, Yu KH, Woung PS. Comment: esomeprazole-induced central fever with severe myalgia. Ann Pharmacother 2005;39(10):1764; author reply 65.
Schonhofer PS, Werner B, Troger U. Ocular damage associated with proton pump inhibitors. BMJ. 1997;314(7097):1805.
Burlinson B, Morriss SH, Gatehouse DG, et al. Genotoxicity studies of gastric acid inhibiting drugs. Lancet. 1990;335(8686):419–20.
National Report on Medicines use in Italy. 2015. The Medicines Utilisation Monitoring Centre. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/Rapporto_OsMed_2015__AIFA.pdf. Last accessed on 23 Sept 2016.
Li XQ, Andersson TB, Ahlstrom M, et al. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32(8):821–7.
Zvyaga T, Chang SY, Chen C, et al. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metab Dispos. 2012;40(9):1698–711.
Desta Z, Zhao X, Shin JG, et al. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41(12):913–58.
Pauli-Magnus C, Rekersbrink S, Klotz U, et al. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedebergs Arch Pharmacol. 2001;364(6):551–7.
Holtzman CW, Wiggins BS, Spinler SA. Role of P-glycoprotein in statin drug interactions. Pharmacotherapy. 2006;26(11):1601–7.
Barkas F, Elisaf M, Rizos CV, et al. Proton pump inhibitors and statins: a possible interaction that favors low-density lipoprotein cholesterol reduction? Hippokratia. 2015;19(4):332–7.
Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80(6):565–81.
Cronican AA, Fitz NF, Pham T, et al. Proton pump inhibitor lansoprazole is a nuclear liver X receptor agonist. Biochem Pharmacol. 2010;79(9):1310–6.
Namazi MR, Sharifian M. The potential anti-xanthoma and anti-atherosclerotic effects of proton pump inhibitors. J Clin Pharm Ther. 2008;33(6):579–80.
Bergvall T, Noren GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37(1):65–77.
Sundstrom A, Hallberg P. Data mining in pharmacovigilance–detecting the unexpected: the role of index of suspicion of the reporter. Drug Saf. 2009;32(5):419–27.
Ferrajolo C, Capuano A, Trifiro G, et al. Pediatric drug safety surveillance in Italian pharmacovigilance network: an overview of adverse drug reactions in the years 2001–2012. Expert Opin Drug Saf. 2014;13(Suppl 1):S9–20.
O’Leary C, McCarthy J, Humphries M, et al. The prophylactic use of a proton pump inhibitor before food and alcohol. Aliment Pharmacol Ther. 2003;17(5):683–6.
Sutherland D, Stanley AJ. Editorial: proton pump inhibitors in cirrhosis. Aliment Pharmacol Ther. 2015;41(6):592.
Lodato F, Azzaroli F, Di Girolamo M, et al. Proton pump inhibitors in cirrhosis: tradition or evidence based practice? World J Gastroenterol. 2008;14(19):2980–5.
Munson JC, Wahl PM, Daniel G, et al. Factors associated with the initiation of proton pump inhibitors in corticosteroid users. Pharmacoepidemiol Drug Saf. 2012;21(4):366–74.
Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315–21.
Noren GN, Bate A, Orre R, et al. Extending the methods used to screen the WHO drug safety database towards analysis of complex associations and improved accuracy for rare events. Stat Med. 2006;25(21):3740–57.
Noren GN, Sundberg R, Bate A, et al. A statistical methodology for drug–drug interaction surveillance. Stat Med. 2008;27(16):3057–70.
Strandell J, Bate A, Hagg S, et al. Rhabdomyolysis a result of azithromycin and statins: an unrecognized interaction. Br J Clin Pharmacol. 2009;68(3):427–34.
Strandell J, Caster O, Bate A, et al. Reporting patterns indicative of adverse drug interactions: a systematic evaluation in VigiBase. Drug Saf. 2011;34(3):253–66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was used for the preparation of this manuscript.
Conflicts of interest
Manfred Hauben is a full-time employed of Pfizer Inc. and receives part of his compensation in the form of stock options and restricted stock units. He has also declared he owns stocks in other pharmaceutical companies that, like Pfizer Inc., manufacture and/or market statins and PPIs. Marco Tuccori, Alice Capogrosso Sansone, Irma Convertino, Maria Teresa Galiulo, Stefano Salvadori, Stefania Pieroni, Tamara Knezevic, Stefania Mantarro, Alessandra Marino and Corrado Blandizzi have no conflicts of interest that are directly relevant to the content of this study.
Ethical Approval and Patient Consent
Ethical approval and patient consent are not required for this kind of study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Capogrosso Sansone, A., Convertino, I., Galiulo, M.T. et al. Muscular Adverse Drug Reactions Associated with Proton Pump Inhibitors: A Disproportionality Analysis Using the Italian National Network of Pharmacovigilance Database. Drug Saf 40, 895–909 (2017). https://doi.org/10.1007/s40264-017-0564-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-017-0564-8