Abstract
Background
Observational data may inform novel drug development programs by identifying previously unappreciated, clinical benefits of existing drugs. Several preclinical and clinical studies have suggested emergent therapeutic utility of drugs acting on the N-methyl-d-aspartate (NMDA) receptor, a subtype of glutamate receptors, including the antidementia drug memantine.
Methods
Using a self-controlled cohort study design, the association of exposure to the NMDA receptor antagonist memantine with the incidence of all observed disease outcomes in four US administrative claims databases, spanning from January 2000 through January 2019, was assessed. The databases used in this study were the IBM MarketScan® Commercial Database (CCAE), the IBM MarketScan® Multi-State Medicaid Database (MDCD), the IBM MarketScan® Medicare Supplemental Database (MDCR), and the Optum© De-Identified Clinformatics® Data Mart Database. Outcomes were defined according to the unique Systematized Nomenclature of Medicine–Clinical Terms (SNOMED CT) classification system codes and required a diagnosis on two or more distinct dates. Of 20,953 outcomes assessed, only those for which memantine was associated with a ≥ 50% reduction in risk in two or more databases were included. A meta-analysis with random effects was used to pool data across the databases.
Results
Overall, 312,336 patients were exposed to memantine during the study. After removing conditions related to dementia and memory loss, 60 outcomes met the threshold criteria. Results fell into five disease categories: mental disorders, substance use disorders, pain, gastrointestinal and colon disorders, and demyelinating disease. The bulk of findings fell into the first two groups, with 28 outcomes related to mental disorders and 24 related to substance use disorders.
Conclusion
The present results confirm that NMDA receptor antagonism may have broader therapeutic utility than previously recognized. Further observational and clinical research may be warranted to explore the therapeutic benefit of NMDA antagonists for the outcomes found in this study.
Similar content being viewed by others
Observational data can be used to identify unknown benefits of existing drugs in order to aid new drug discovery and development. |
This study found large protective associations between memantine, an NMDA receptor antagonist, and conditions related to mood and psychotic disorders, substance use disorders, pain, multiple sclerosis, and gastrointestinal/colon disorders. |
The results suggest that NMDA receptor inhibition may have protective effects in multiple therapeutic areas, which could aid drug discovery. |
1 Introduction
More than 2000 unique drug ingredients are currently being prescribed in the US, with many approved for a single indication. An enormous opportunity exists to leverage existing health outcomes data from real-world use of these medications to identify potentially new uses for these drugs, as well as to develop similar medications targeted for new therapeutic uses.
Memantine is an N-methyl-d-aspartate (NMDA) receptor antagonist, a subset of glutamate receptors, and its real-world use can be studied to identify unknown benefits of therapies that affect the NMDA pathway. Memantine is indicated for the treatment of moderate-to-severe dementia associated with Alzheimer’s disease and was approved by the US FDA in 2003 [1], the European Medicines Agency in 2002 [2], and also by other global regulatory agencies. The drug has an established safety profile [3, 4] and has been hypothesized to be an efficacious therapy for numerous disease areas beyond dementia and Alzheimer’s disease, including mood disorders, neurodegenerative diseases, pain, drug dependence, and others [5]. Evidence from preclinical and clinical studies examining some of these off-label uses has been mixed [6, 7].
The use of administrative health insurance claims data can aid in the process of discovering new treatments by uncovering unknown benefits of existing medications. These claims data capture medications filled by patients and the conditions diagnosed by their physicians, among other information, and can help inform therapeutic development and new drug discovery if used appropriately [8,9,10]. Claims data already play a pivotal role in studying the safety and effectiveness of treatments [11,12,13]; however, there is much broader potential for their use. By harnessing these real-world data, we may provide insights into unknown benefits of existing drugs and better understand disease etiology and prevention, which may inform novel drug development programs.
We have built upon advances in statistical methodologies and computing power to create the real-world assessment and research of drug performance (REWARD) framework for studying the association between thousands of medications with thousands of outcomes in millions of patients. Prior work leveraging this framework has focused on specific outcomes of interest to identify heretofore unappreciated uses of existing drugs to treat or prevent a specific disease [14, 15]. In this study, we have taken the reverse approach, by examining exposure to a single drug, memantine, and measuring its association with all potential outcomes, in order to identify potential indications for new NMDA receptor antagonist therapies. This work represents the first step of the drug discovery process, i.e. hypothesis generation, which may be used to inform future clinical research.
2 Methods
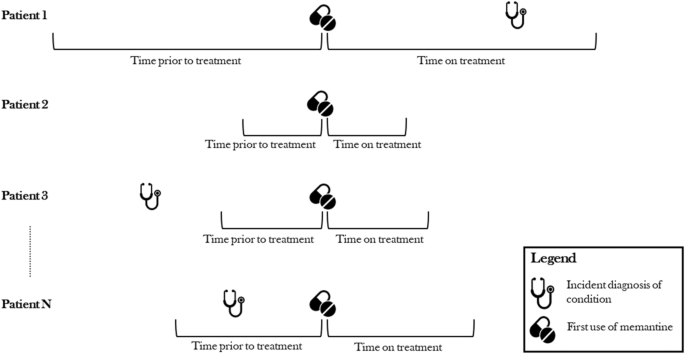
This study leveraged a self-controlled cohort design in which individuals served as their own controls. Self-controlled study designs tend to produce less biased estimates with higher predictive accuracy than other, more commonly used designs, such as case-control studies [16]. A separate analysis was performed for every condition in each database (see the Sect. 2.1 below) to determine its association with the use of memantine. Figure 1 illustrates the study design for a single condition outcome. The REWARD framework has been used in previous studies examining treatments associated with the prevention of parkinsonism and Alzheimer’s disease [14, 15].
Self-controlled cohort study design. An example studying the association between memantine and incidence of a single outcome by including all patients who were exposed to the medication. It shows the condition first occurring during the time a patient was on treatment (Patient 1), not occurring in a patient’s history (Patient 2), occurring outside of the observation windows and therefore not counted in either period (Patient 3), and occurring during the unexposed control period (Patient N). This approach was repeated for all 20,953 conditions identified in the database
2.1 Data Sources
The analysis was executed in four US-based administrative claims databases. Each database contains data from adjudicated health insurance claims (e.g. inpatient, outpatient/emergency department, and outpatient pharmacy) and health plan enrollment information. Briefly, the four databases included in this study were as follows.
-
1.
IBM MarketScan® Commercial Database (CCAE): Includes data from 144 million individuals enrolled in employer-sponsored insurance health plans. Data spanned 1 January 2000 through 31 January 2019.
-
2.
IBM MarketScan® Multi-State Medicaid Database (MDCD): A claims database for 28 million Medicaid enrollees from multiple states. Data spanned 1 January 2006 through 30 June 2018.
-
3.
IBM MarketScan® Medicare Supplemental Database (MDCR): Includes data for more than 10 million retirees with primary or Medicare supplemental coverage through privately insured fee-for-service, point-of-service, or capitated health plans. Data spanned 1 January 2000 through 31 January 2019.
-
4.
Optum © De-Identified Clinformatics® Data Mart Database. Includes 85 million members with private health insurance who are fully insured in commercial plans or Medicare Advantage. Data spanned 1 May 2000 through 31 December 2018.
Data elements included were outpatient pharmacy dispensing claims (coded with National Drug Codes), inpatient and outpatient medical claims that provide diagnosis codes (coded in the International Classification of Diseases [ICD], Ninth Revision, Clinical Modification [ICD-9-CM] or ICD Tenth Revision, Clinical Modification [ICD-10-CM]) associated with a visit. The use of the IBM MarketScan and Optum claims databases was reviewed by the New England Institutional Review Board (IRB) and was determined to be exempt from broad IRB approval, as this research project did not involve human subjects research.
2.2 Exposure and Control Definition
The exposure of interest was the medication memantine, which was identified according to the RxNorm ingredient (#6719) [17]. Individuals were identified at the time they first filled a prescription for memantine. An exposure period was defined as the period starting with initiation of the medication until discontinuation or end of observation, whichever came first. Continuous medication use allowed for a maximum gap between consecutive fills equal to the medication supply plus 30 days. The time directly preceding the exposure, and equal in length to the exposure period, served as the unexposed (i.e. control) period. If the time preceding the exposure was less than the time exposed, the exposure period was truncated to match the available unexposed period to maintain an equal amount of observation for both periods.
2.3 Outcome Definition
Outcomes were defined for every condition present in the Observational Medical Outcomes Partnership (OMOP) Common Data Model v5.0 [12] according to the Systematized Nomenclature of Medicine–Clinical Terms (SNOMED CT) classification system codes. The SNOMED CT classification allows mapping of various diagnostic languages, including ICD-9-CM and ICD-10-CM, to a single standardized set of concepts, and is used by the common data model leveraged for the present study [18]. An incident outcome of a condition required a diagnosis for the condition on at least two distinct dates. The date of the first diagnosis was considered the outcome date. Overall, 20,953 distinct conditions were examined. Outcomes were grouped into broad disease categories for reporting purposes.
Results that were related to dementia and Alzheimer’s disease, the primary indication of memantine, were excluded, as were conditions thought to be a cause or symptom of dementia, such as stroke and other cerebrovascular disease, cancers of the central nervous system, problems with cognition and memory, sleep disorders, and falls and fractures. These were excluded because the results could be biased due to the study design; for example, outcomes that cause dementia will primarily occur before starting treatment for dementia and will thus appear protective. Conditions that are typically diagnosed in younger individuals, such as autism, which would not be expected to be first diagnosed in a patient who is being prescribed a dementia treatment, were also excluded.
2.4 Statistical Analysis
The incidence rates (IR) of all outcomes were calculated for the exposed and unexposed periods of memantine treatment. IRe is defined as the number of all individuals with the condition first diagnosed during the exposed period divided by the sum of exposed time across all patients. Similarly, IRu was calculated as the number of all individuals with an incident diagnosis during the unexposed period divided by the sum of unexposed time, which is equal to the sum of exposed time, across all exposed patients. An incident rate ratio (IRR) was then calculated as IRe divided by IRu. If there is no association between memantine and the condition, the expectation is that cases of the condition will be equally distributed prior to and following the initiation of memantine, leading to an IRR of 1.0. An IRR > 1.0 indicates more cases identified after initiation of memantine, while an IRR < 1.0 indicates fewer cases identified after initiation. Poisson models were used to model the IRRs for each condition outcome and to test for statistical significance (IRR ≠ 1.0, with α = 0.05) using the methods developed by Graham et al. [19, 20].
Two strict criteria were applied to identify conditions for which memantine has potential protective effects.
-
1.
Memantine must have been associated with at least a 50% reduction in the incidence of the condition (i.e. IRR ≤ 0.5) in at least two of the four databases, with p < 0.05.
-
2.
There must have been no evidence of the contrary association of significant risk between memantine and the condition in any of the databases, defined as having an IRR > 2.0, with p < 0.05.
The IRRs and 95% confidence intervals (CIs) were reported for analyses in each of the four databases. A meta-analysis with random effects was then performed to pool results across the four databases into a single effect estimate and 95% CI. The Mantel–Haenszel method was used to pool effect estimates, and between-study variance was estimated using the maximum-likelihood estimator. The I2 measure was used to measure heterogeneity of the associations across the data sources.
3 Results
Overall, 312,336 patients were exposed to memantine during the study period. Average patient exposure durations to memantine varied by outcome, ranging from 422 days for patients diagnosed with demyelinating disease, up to 623 days for diverticulitis of the colon (Table 1). From the 20,000+ conditions assessed, and after removing those related to dementia and memory loss, there were 60 outcomes that met the threshold criteria of having a statistically significant benefit of at least a 50% risk reduction in two or more of the four databases. Results fell into five main disease categories: mental disorders, substance use disorders, pain, gastrointestinal (GI) and colon disorders, and demyelinating disease. The bulk of significant findings fell into the first two groups, with 28 outcomes related to mental disorders and 24 related to drug/alcohol abuse. For brevity of reporting purposes, up to three outcomes from each category are presented here and also in Table 1. A forest plot of the results from the meta-analysis is shown in Fig. 2. Full results of all significant outcomes within each of the four databases are shown in electronic supplementary Table 1.
Within the ‘mental disorder’ category, the most commonly observed outcome was ‘mood disorder’; however, because it is a high-level term in the SNOMED hierarchy and contains many of the other findings, we focused on the more specific outcomes of ‘depressive disorder’, ‘psychotic disorder’, and ‘bipolar disorder’. Combining results across all four databases resulted in a 47% reduced risk of the outcome of depressive disorder (meta-analysis IRR 0.53, 95% CI 0.45–0.61). The association with bipolar disease was similar to depression when including data across all databases (meta-analysis IRR 0.52, 95% CI 0.39–0.70), while a slightly stronger effect was found for psychotic disorder, with a 57% reduced risk (meta-analysis IRR 0.43, 95% CI 0.37–0.49).
The most common significant outcome in the alcohol and drug abuse class was ‘drug dependence’. Memantine was associated with a more than 50% reduced risk in the meta-analysis (IRR 0.48, 95% CI 0.41–0.57). Other common outcomes in this group include ‘psychoactive substance use disorder’ (meta-analysis IRR 0.46, 95% CI 0.41–0.52) and ‘alcoholism’ (meta-analysis IRR 0.44, 95% CI 0.40–0.50).
For pain, the three findings that were not generic (i.e. ‘pain’ and ‘pain finding at anatomical site’) were ‘pain of truncal structure’, ‘idiopathic peripheral neuropathy’, and ‘atypical face pain’. Associations with ‘pain of truncal structure’ were consistent across all databases, with IRRs ranging from 0.46 to 0.49. The IRR from the meta-analysis was 0.47 (95% CI 0.45–0.49), indicating a 53% reduced risk while taking memantine. The association with idiopathic peripheral neuropathy showed a 45% reduced risk (95% CI 36–53%) across all databases. The outcome of atypical facial pain was much less common than the other outcomes but showed strong associations across the databases, with a meta-analysis IRR of 0.35 (95% CI 0.23–0.54).
Significant GI and colon disorders include ‘diverticulosis of colon’ and ‘stenosis of intestine’. Diverticulitis of the colon was associated with a 46% reduced risk across all databases (95% CI 37–54%). Stenosis of the intestine occurred in just eight patients while they were exposed to memantine and 29 patients prior to exposure, resulting in a meta-analysis IRR of 0.27 (95% CI 0.12–0.59), a 73% reduction in risk.
The single finding for demyelinating diseases included the SNOMED concept of ‘demyelinating disease of central nervous system’, which is accounted for almost entirely by multiple sclerosis (MS). Strong associations were observed across databases, with a nearly two-thirds reduction in risk (meta-analysis IRR 0.36, 95% CI 0.24–0.54).
4 Discussion
We examined the association between memantine and more than 20,000 incident condition outcomes across four US administrative claims databases in more than 300,000 users of the drug. This analysis identified 60 outcomes for which memantine showed a strong, consistent, protective association, falling into five main groups: mental disorders, substance use disorders, pain, GI and colon disorders, and demyelinating disease. The results of this study are specific to the drug memantine; however, the aim was to generate hypotheses for future development of brand-new NMDA receptors, rather than identifying indications for potential repurposing of memantine.
We found that memantine was associated with a decreased risk of depression and bipolar disease. These findings are not entirely unexpected as glutamate levels have been found to be increased in the occipital cortex of patients with major depression [21]; however, there has been a lack of any further evidence of this association in the last 16 years. Additionally, glutamate binds to NMDA receptors, and esketamine, an NMDA receptor antagonist, has proven effective for the treatment of treatment-resistant depression and major depressive disorder with acute suicidal ideation or behavior [22, 23]. While our findings support the potential preventive effects of memantine in depression, bipolar disorder, and other mental health disorders, previous clinical studies have shown mixed evidence on the efficacy of memantine for the treatment of these conditions [24,25,26,27,28,29,30,31,32]. There are many potential reasons for the differences in evidence of prior preclinical and clinical work. One possible explanation is that the differences may reflect the time course of NMDA receptor involvement in the etiology, progression, and/or maintenance of these diseases, as well as the limited target selectivity of the NMDA receptor antagonist.
The other largest group of significant outcomes in the present study was also related to mental health. Memantine was found to be protective against multiple substance use disorders, including abuse and dependence related to alcohol, nicotine, and other psychoactive substances. Consistent with our findings, results from previous preclinical and clinical research have shown memantine to be useful in preventing relapses in opioid-dependent patients [33], reduced the feelings of being ‘buzzed’ after smoking [34], and prevented the acquisition of nicotine in rats [34]. Results from trials studying the efficacy of memantine on alcohol dependence were also mixed [35, 36]. The findings presented here provide evidence for a potential role of NMDA receptor antagonists for the treatment of substance use disorders.
This study also found memantine to have protective effects for pain-related outcomes, such as peripheral neuropathy. NMDA receptor antagonists, and ketamine in particular, have been previously studied for their potential pain management benefits [37, 38]. A recent systematic literature review examined the effects of memantine on neuropathic pain and identified prophylactic effects against postoperative neuralgia and pain-associated psychological impairment [37]. Furthermore, the results of a preclinical animal study [39] point to memantine as a potential treatment for preventing neuropathic pain.
The last of our findings relate to the potential protective effects for MS and GI/colon-related conditions. Animal studies suggest that NMDA receptor antagonists have a stabilizing effect on the blood–brain barrier and brain inflammatory response, and exhibit neuroprotective properties [40], and it has been hypothesized that NMDA antagonism can reduce neuronal damage in MS [41]. However, a randomized clinical trial found no effect of memantine on outcomes related to MS disease severity compared with placebo [42]. The observed potential benefit of memantine on diverticulosis could be due to its neuroprotective effects, decreasing the neural damage and regeneration that is observed in subjects with diverticular disease [43, 44].
There are multiple strengths of this study. For example, allowing patients to serve as their own control eliminated potential confounds from both observed and unobserved time-invariant covariates (e.g. genetics). Moreover, the study described here utilized 83,812 different statistical models, examining the association of memantine with every potential disease outcome across four distinct databases. We used strict filtering criteria to pare down such a large number of results and avoid erroneous findings due to multiple comparisons and narrow CIs. Our filters included the requirement that memantine must have been associated with at least a 50% reduction in the incidence of an outcome, and the association must have been replicated in at least one of the other three databases. While type 1 error was not a major concern of this study, as the primary goal was to generate testable hypotheses regarding the potential development of new NMDA antagonists for treating novel indications, there is still a possibility of ‘false positives’ due to misclassification of the exposure and/or outcomes in the claims data, confounding variables such as concomitant medication use, or pure chance.
The use of strict filtering criteria and minimization of false positives has a downside, in that outcomes for which memantine shows a potential benefit but did not meet the high bar for inclusion may have been missed. Memantine has been hypothesized to protect against age-related macular degeneration via its ability to significantly decrease calcium channel activity [45], but this outcome was not found to be significant in our study. When the results were examined in more detail, there was an association with the incidence of age-related macular degeneration in three of the four databases and a meta-analysis IRR of 0.67 (95% CI 0.62–0.73). This illustrates an example of a potential ‘miss’ of a true association due to overly strict filters, or it may be that there truly is no association and the outcome was properly excluded, i.e. the filters were doing their job.
Thus, the absence of significant findings do not necessarily mean that there is truly no association, but rather that any observed association was not strong enough to be included.
On the other hand, a limitation of the self-controlled cohort design is that it does not control for time-variant confounding, such as age or concomitant medication use. Because observations were limited to a concise period during which patients were being treated and the time immediately prior, the effects of these biases were partially mitigated; however, any immediate changes that occurred during this period, especially those that occurred at the same time the patient started taking memantine, could confound the results. Another important limitation to this study design is the effect of temporal sequencing of disease, treatment, and comorbid or causal conditions. For example, memantine is used to treat dementia, which may be caused by an ischemic stroke. In this case, stroke precedes both the onset of dementia and the treatment of memantine. In our study design, memantine would have demonstrated protection against stroke, because the outcome of stroke occurs more often prior to initiation of the exposure than it does after, independent of whether there is any direct causality between memantine and stroke. To avoid reporting such erroneous findings, conditions where we believed this temporal bias was occurring were removed. Similarly, off-label use of memantine for other conditions could bias the findings to show protective effects for these conditions. Off-label use has recently been studied [46], including the self-medication with memantine to treat conditions such as anxiety, depression, attention-deficit hyperactivity disorder, and obsessive-compulsive disorder. While such use could lead to bias in our results, it is unlikely to be a common enough behavior to account for the majority of the observed effect.
A final limitation is the reliance on administrative insurance claims data. However, we leveraged the standardized SNOMED CT classification system to define our outcomes, rather than use our own subjective definition of conditions. To avoid false positive outcomes due to a single exclusion or misdiagnosis, we required a diagnosis for the same condition to occur on at least two distinct dates. While the use of multiple diagnoses for the same condition should limit the number of ‘rule-out’ diagnoses picked up by the data, it does not correct for systematic incorrect coding of a condition throughout a patient’s history.
5 Conclusion
This work illustrates the use of real-world data to identify potential benefits of the NMDA receptor antagonist memantine, which could be used to identify indications for the development of brand-new NMDA receptor antagonists. Identifying new benefits of existing medications, such as memantine, may allow us to address large unmet needs in diseases where treatment options are currently limited, or in patients where existing treatments have failed. We uncovered 60 diseases for which memantine may be protective, mostly conditions related to mood and psychotic disorders, substance use disorders, and pain. The results of this study may be used to generate hypotheses for future observational, preclinical, and clinical research examining the efficacy of inhibiting the NMDA pathway in preventing or treating these conditions.
References
Forest Pharmaceuticals Inc. NAMENDA (memantine HCl) [package insert]. US FDA; 2003. Revised October 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021487s010s012s014,021627s008lbl.pdf. Accessed 6 Aug 2020.
European Medicines Agency. Ebixa (memantine). 2020 [cited 14 Dec 2020]. https://www.ema.europa.eu/en/medicines/human/EPAR/ebixa.
Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–41.
Ferris SH. Evaluation of memantine for the treatment of Alzheimer’s disease. Expert Opin Pharmacother. 2003;4(12):2305–13.
Rammes G, Danysz W, Parsons CG. Pharmacodynamics of memantine: an update. Curr Neuropharmacol. 2008;6(1):55–78.
Zdanys K, Tampi RR. A systematic review of off-label uses of memantine for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1362–74.
Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias. CNS Drugs. 2012;26(8):663–90.
Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–7.
Singh G, Schulthess D, Hughes N, Vannieuwenhuyse B, Kalra D. Real world big data for clinical research and drug development. Drug Discov Today. 2018;23(3):652–60.
Yao L, Zhang Y, Li Y, Sanseau P, Agarwal P. Electronic health records: implications for drug discovery. Drug Discov Today. 2011;16(13):594–9.
Berger ML, Sox H, Willke RJ, Brixner DL, Eichler HG, Goettsch W, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the Joint ISPOR-ISPE Special Task Force on Real-World Evidence in Health Care Decision Making. Value Health. 2017;20(8):1003–8.
Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, Reich C, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med. 2010;153(9):600–6.
Corrigan-Curay J, Sacks L, Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320(9):867–8.
Kern DM, Cepeda MS, Lovestone S, Seabrook GR. Aiding the discovery of new treatments for dementia by uncovering unknown benefits of existing medications. Alzheimer’s Dement Transl Res Clin Interv. 2019;5:862–70.
Cepeda MS, Kern DM, Seabrook GR, Lovestone S. Comprehensive real-world assessment of marketed medications to guide parkinson’s drug discovery. Clin Drug Investig. 2019;39(11):1067–75.
Ryan PB, Stang PE, Overhage JM, Suchard MA, Hartzema AG, DuMouchel W, et al. A comparison of the empirical performance of methods for a risk identification system. Drug Saf. 2013;36(Suppl 1):S143–58.
US National Library of Medicine. Unified Medical Language System® (UMLS®): RxNorm. 2019. https://www.nlm.nih.gov/research/umls/rxnorm/.
SNOMED CT. SNOMED CT 5-Step Briefing. 2019. http://www.snomed.org/snomed-ct/five-step-briefing.
Ryan PB, Schuemie MJ, Madigan D. Empirical performance of the case-control method: lessons for developing a risk identification and analysis system. Drug Saf. 2013;36:95–106.
Graham PL, Mengersen K, Morton AP. Confidence limits for the ratio of two rates based on likelihood scores: non-iterative method. Stat Med. 2003;22(12):2071–83.
Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, et al. Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61(7):705–13.
Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139–48. https://doi.org/10.1001/jamapsychiatry.2017.3739.
Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression—first FDA-approved antidepressant in a new class. N Engl J Med. 2019;381(1):1–4.
Agarwal V, Tripathi A. Memantine in the management of a clinically challenging case of bipolar disorder. Indian J Psychiatry. 2009;51(2):137–8.
Strzelecki D, Tabaszewska A, Barszcz Z, Józefowicz O, Kropiwnicki P, Rabe-Jabłońska J. A 10-week memantine treatment in bipolar depression: a case report. Focus on depressive symptomatology, cognitive parameters and quality of life. Psychiatry Investig. 2013;10(4):421–4.
Koukopoulos A, Serra G, Koukopoulos AE, Reginaldi D, Serra G. The sustained mood-stabilizing effect of memantine in the management of treatment resistant bipolar disorders: findings from a 12-month naturalistic trial. J Affect Disord. 2012;136(1):163–6.
Krause-Sorio B, Siddarth P, Kilpatrick L, Laird KT, Milillo MM, Ercoli L, et al. Combined treatment with escitalopram and memantine increases gray matter volume and cortical thickness compared to escitalopram and placebo in a pilot study of geriatric depression. J Affect Disord. 2020;274:464–70.
Kishi T, Matsunaga S, Iwata N. A meta-analysis of memantine for depression. J Alzheimers Dis. 2017;57(1):113–21.
Feusner JD, Kerwin L, Saxena S, Bystritsky A. Differential efficacy of memantine for obsessive-compulsive disorder vs. generalized anxiety disorder: an open-label trial. Psychopharmacol Bull. 2009;42(1):81–93.
Kulkarni J, Thomas N, Hudaib A-R, Gavrilidis E, Grigg J, Tan R, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind placebo-controlled trial. CNS Drugs. 2018;32(2):179–87.
Tavakoli-Ardakani M, Abbaspour H, Farhadi Nasab A, Mazaheri Meibodi A, Kheradmand A. Study of the effect of memantine on negative sign in patients with schizophrenia and schizoaffective disorders. Iran J Pharm Res. 2018;17(Suppl):122–9.
Lieberman JA, Papadakis K, Csernansky J, Litman R, Volavka J, Jia XD, et al. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacol. 2009;34(5):1322–9.
Popik P, Wrobel M, Bisaga A. Reinstatement of morphine-conditioned reward is blocked by memantine. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2006;31(1):160–70.
Jackson A, Nesic J, Groombridge C, Clowry O, Rusted J, Duka T. Differential involvement of glutamatergic mechanisms in the cognitive and subjective effects of smoking. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2009;34(2):257–65.
Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin Exp Res. 2007;31(5):775–82.
Krishnan-Sarin S, O’Malley SS, Franco N, Cavallo DA, Tetrault JM, Shi J, et al. Influence of combined treatment with naltrexone and memantine on alcohol drinking behaviors: a phase II randomized crossover trial. Neuropsychopharmacology. 2020;45(2):319–26.
Thompson T, Whiter F, Gallop K, Veronese N, Solmi M, Newton P, et al. NMDA receptor antagonists and pain relief: a meta-analysis of experimental trials. Neurology. 2019;92(14):e1652–62.
Kreutzwiser D, Tawfic QA. Expanding role of NMDA receptor antagonists in the management of pain. CNS Drugs. 2019;33(4):347–74.
Morel V, Etienne M, Wattiez A-S, Dupuis A, Privat A-M, Chalus M, et al. Memantine, a promising drug for the prevention of neuropathic pain in rat. Eur J Pharmacol. 2013;721(1):382–90.
Paul C, Bolton C. Modulation of blood-brain barrier dysfunction and neurological deficits during acute experimental allergic encephalomyelitis by the N-methyl-d-aspartate receptor antagonist memantine. J Pharmacol Exp Ther. 2002;302(1):50–7.
Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1(1):101–10.
Peyro Saint Paul L, Creveuil C, Heinzlef O, De Seze J, Vermersch P, Castelnovo G, et al. Efficacy and safety profile of memantine in patients with cognitive impairment in multiple sclerosis: a randomized, placebo-controlled study. J Neurol Sci. 2016;363:69–76.
Tursi A, Elisei W. Role of inflammation in the pathogenesis of diverticular disease. Mediat Inflamm. 2019;2019:8328490. https://doi.org/10.1155/2019/8328490 ((Fric J (academic editor))).
Simpson J, Sundler F, Jenkins D, Spiller RC. Increased expression of galanin in mucosal nerves of patients with painful diverticular disease. Clin Sci. 2003;104(s49):35P.
Bardak H, Uğuz AC, Bardak Y. Protective effects of melatonin and memantine in human retinal pigment epithelium (ARPE-19) cells against 2-ethylpyridine-induced oxidative stress: implications for age-related macular degeneration. Cutan Ocul Toxicol. 2018;37(2):112–20.
Natter J, Michel B. Memantine misuse and social networks: a content analysis of Internet self-reports. Pharmacoepidemiol Drug Saf. 2020. https://doi.org/10.1002/pds.5070.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was received for this study. Janssen Research & Development paid the open access fees.
Conflict of interest
David M. Kern, M. Soledad Cepeda, Christopher M. Flores, and Gayle M. Wittenberg are employees of Janssen Research & Development.
Availability of data and material
The dataset of full study results of all significant outcomes can be found in the electronic supplementary material.
Ethics approval
The use of the IBM MarketScan and Optum claims databases was reviewed by the New England IRB and was determined to be exempt from broad IRB approval as this research project did not involve human subjects research.
Consent for publication
Not applicable.
Author contributions
DMK and MSC designed the study and conducted the analysis. DMK, MSC, CMF and GW interpreted the results. DMK wrote the manuscript, and MSC, CMF, and GW critically revised the work. All authors contributed to the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kern, D.M., Cepeda, M.S., Flores, C.M. et al. Application of Real-World Data and the REWARD Framework to Detect Unknown Benefits of Memantine and Identify Potential Disease Targets for New NMDA Receptor Antagonists. CNS Drugs 35, 243–251 (2021). https://doi.org/10.1007/s40263-020-00789-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00789-3