Abstract
Background
The necessity of annual laboratory follow-up in patients treated with valproic acid (VPA) is controversial.
Objective
We investigated the need for annual laboratory follow-up of liver enzymes, electrolytes, and full blood count (FBC) in patients treated with VPA.
Patients and methods
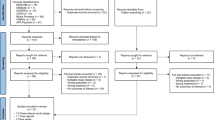
A systematic search in Evidence-Based Medicine Reviews (EBMR), MEDLINE, and EMBASE was undertaken in December 2016 to identify all published articles investigating or citing valproic acid, liver function disorders, electrolyte disorders, and FBC deviations.
Results
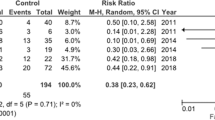
This review included 108 articles. As the number of participants and duration of the study was not adequate in most studies to detect rare adverse events, studies did not demonstrate a clear prevalence of hepatotoxicity. While a transient increase of transaminases is common and seldom harmful, severe hepatotoxicity is a rare phenomenon and is not prevented by routine laboratory monitoring. VPA had no relevant effect on serum calcium, sodium, potassium, and albumin. The prevalence of FBC varied from 0.6 to 27.8%, occurred mostly in the first 2 years of therapy, and was usually asymptomatic.
Conclusions
Long-term monitoring in VPA treatment is only necessary when there have been dose adjustments, co-medication switches, or co-morbidity. In uncomplicated cases, annual laboratory follow-up may be discontinued after 2 years of VPA treatment. Encouraging patients to be vigilant is more effective in the detection of hepatotoxicity than laboratory testing. Follow-up of FBC at 3–6 months, 1 year, and 2 years after start or after a dose increase of VPA or interacting medication is sufficient.

Similar content being viewed by others
References
Ng F, Mammen OK, Wilting I, Sachs GS, Ferrier IN, Cassidy F, Beaulieu S, Yatham LN, Berk M, International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559–95.
NICE guideline. Bipolar disorder: assessment and management. https://www.nice.org.uk/guidance/cg185/chapter/1-Recommendations#how-to-use-medication. Accessed 31 May 2017.
Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495–553.
Kupka R, Goossens P, van Bendegem M, Daemen P, Daggenvoorde T, Daniels M, Dols A, Hillegers M, Hoogelander A, ter Kulve E, Peetoom T, Schulte R, Stevens A, van Duine D. Multidisciplinaire richtlijn bipolaire stoornissen. Utrecht: De Tijdstroom; 2015. p. 167–73.
NICE guideline. Epilepsies: diagnosis and management. https://www.nice.org.uk/guidance/cg137. Accessed 28 Jun 2017.
Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, O’Donovan C, Macqueen G, McIntyre RS, Sharma V, Ravindran A, Young LT, Milev R, Bond DJ, Frey BN, Goldstein BI, Lafer B, Birmaher B, Ha K, Nolen WA, Berk M. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1–44.
Fenichel GM, Greene HL. Valproate hepatotoxicity: two new cases, a summary of others, and recommendations. Pediatr Neurol. 1985;1(2):109–13.
Goto S, Seo T, Hagiwara T, et al. Potential relationships between transaminase abnormality and valproic acid clearance or serum carnitine concentrations in Japanese epileptic patients. J Pharm Pharmacol. 2008;60(2):267–72.
Cimino C, Charneski L, Kumar L. Idiosyncratic valproic acid-induced hepatotoxicity in a sickle cell patient. J Pharm Technol. 2015;31(1):43–4.
Pinkston R, Walker LA. Multiorgan system failure caused by valproic acid toxicity. Am J Emerg Med. 1997;15(5):504–6.
SSPC Depakote product information. https://www.medicines.org.uk/emc/medicine/25929. Accessed 16 May 2017.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;21(339):b2535.
Cepelak I, Zanić Grubisić T, Mandusić A, Rekić B, Lenicek J. Valproate and carbamazepine comedication changes hepatic enzyme activities in sera of epileptic children. Clin Chim Acta. 1998;276(2):121–7.
Hayes JF, Marston L, Walters K, Geddes JR, King M, Osborn DP. Adverse renal, endocrine, hepatic, and metabolic events during maintenance mood stabilizer treatment for bipolar disorder: a population-based cohort study. PLoS Med. 2016;13(8):e1002058.
Hemingway C, Leary M, Riordan G, Schlegal B, Walker K. The effect of carbamazepine and sodium valproate on the blood and serum values of children from a third-world environment. J Child Neurol. 1999;14(11):751–3.
Attilakos A, Voudris KA, Katsarou E, Prassouli A, Mastroyianni S, Garoufi A. Transient decrease in serum albumin concentrations in epileptic children treated with sodium valproate monotherapy. Clin Neuropharmacol. 2007;30(3):145–9.
Salehiomran MR, Hosseini SE. The effect of anticonvulsant drugs (phenobarbital and valproic acid) on the serum level of cholesterol, triglyceride, lipoprotein and liver enzymes in convulsive children. Iran J Child Neurol. 2010;4(3):33–8.
Jha S, Agarwal DK, Shukla S, Nag D, Saxena RC. Hepatic functions in epileptics on sodium valproate monotherapy. Indian J Physiol Pharmacol. 1995;39(3):305–6.
Amitai M, Sachs E, Zivony A, Remez R, Ben Baruch R, Amit BH, Kronenberg S, Apter A, Shoval G, Weizman A, Zalsman G. Effects of long-term valproic acid treatment on hematological and biochemical parameters in adolescent psychiatric inpatients: a retrospective naturalistic study. Int Clin Psychopharmacol. 2015;30(5):241–8.
Ugras M, Yakinci C. Protein C, protein S and other pro- and anticoagulant activities among epileptic children using sodium valproate. Brain Dev. 2006;28(9):549–53.
Lenz RA, Elterman RD, Robieson WZ, Vigna NV, Saltarelli MD. Divalproex sodium in children with partial seizures: 12-month safety study. Pediatr Neurol. 2009;41(2):101–10.
Hauser E, Seidl R, Freilinger M, Male C, Herkner K. Hematologic manifestations and impaired liver synthetic function during valproate monotherapy. Brain Dev. 1996;18(2):105–9.
Demircioğlu S, Soylu A, Dirik E. Carbamazepine and valproic acid: effects on the serum lipids and liver functions in children. Pediatr Neurol. 2000;23(2):142–6.
Berkovic SF, Bladin PF, Jones DB, Smallwood RA, Vajda FJ. Hepatotoxicity of sodium valproate. Clin Exp Neurol. 1983;19:192–7.
Thomson MA, Lynch S, Strong R, Shepherd RW, Marsh W. Orthotopic liver transplantation with poor neurologic outcome in valproate-associated liver failure: a need for critical risk-benefit appraisal in the use of valproate. Transplant Proc. 2000;32(1):200–3.
Romero-Falcón A, de la Santa-Belda E, García-Contreras R, Varela JM. A case of valproate-associated hepatotoxicity treated with L-carnitine. Eur J Intern Med. 2003;14(5):338–40.
McLaughlin DB, Eadie MJ, Parker-Scott SL, Addison RS, Henderson RD, Hooper WD, Dickinson RG. Valproate metabolism during valproate-associated hepatotoxicity in a surviving adult patient. Epilepsy Res. 2000;41(3):259–68.
Colletti RB, Trainer TD, Krawisz BR. Reversible valproate fulminant hepatic failure. J Pediatr Gastroenterol Nutr. 1986;5(6):990–4.
Yaman A, Kendirli T, Odek C, Bektaş O, Kuloğlu Z, Koloğlu M, Ince E, Deda G. Valproic acid-induced acute pancreatitis and multiorgan failure in a child. Pediatr Emerg Care. 2013;29(5):659–61.
Mettananda S, Fernando AD, Ginige N. Posterior reversible encephalopathy syndrome in a survivor of valproate-induced acute liver failure: a case report. J Med Case Rep. 2013;31(7):144.
Krähenbühl S, Mang G, Kupferschmidt H, Meier PJ, Krause M. Plasma and hepatic carnitine and coenzyme A pools in a patient with fatal, valproate induced hepatotoxicity. Gut. 1995;37(1):140–3.
König SA, Schenk M, Sick C, Holm E, Heubner C, Weiss A, König I, Hehlmann R. Fatal liver failure associated with valproate therapy in a patient with Friedreich’s disease: review of valproate hepatotoxicity in adults. Epilepsia. 1999;40(7):1036–40.
Chabrol B, Mancini J, Chretien D, Rustin P, Munnich A, Pinsard N. Valproate-induced hepatic failure in a case of cytochrome c oxidase deficiency. Eur J Pediatr. 1994;153(2):133–5.
Huang YL, Hong HS, Wang ZW, Kuo TT. Fatal sodium valproate-induced hypersensitivity syndrome with lichenoid dermatitis and fulminant hepatitis. J Am Acad Dermatol. 2003;49(2):316–9.
Rej S, Chen T, Edwards M, Zicherman V. Fulminant hepatic failure in the context of reinstituting valproate use. Psychosomatics. 2014;55(3):303–4.
Bell EA, Shaefer MS, Markin RS, Wood RP, Langnas AN, Stratta RJ, Shaw BW Jr. Treatment of valproic acid-associated hepatic failure with orthotopic liver transplantation. Ann Pharmacother. 1992;26(1):18–21.
Koenig SA, Buesing D, Longin E, Oehring R, Häussermann P, Kluger G, Lindmayer F, Hanusch R, Degen I, Kuhn H, Samii K, Jungck A, Brückner R, Seitz R, Boxtermann W, Weber Y, Knapp R, Richard HH, Weidner B, Kasper JM, Haensch CA, Fitzek S, Hartmann M, Borusiak P, Müller-Deile A, Degenhardt V, Korenke GC, Hoppen T, Specht U, Gerstner T. Valproic acid-induced hepatopathy: nine new fatalities in Germany from 1994 to 2003. Epilepsia. 2006;47(12):2027–31.
van Zoelen MA, de Graaf M, van Dijk MR, Bogte A, van Erpecum KJ, Rockmann H, Maarschalk-Ellerbroek LJ. Valproic acid-induced DRESS syndrome with acute liver failure. Neth J Med. 2012;70(3):155.
Bauer MS. Fatal hepatic failure and valproate. Am J Psychiatry. 2005;162(1):192.
Puri AS, Sharma BC, Khan EM, Saraswat VA. Fatal fulminant hepatic failure due to sodium valproate in an adolescent. Indian Pediatr. 1994;31(2):207–10.
Parra J, Iriarte J, Pierre-Louis SJ. Valproate toxicity. Neurology. 1996;47(6):1608.
Schmid MM, Connemann BJ, Wolf RC, Flechtenmacher C, Eisenbach C, Schönfeldt-Lecuona C. Can we prevent fatal liver failure under valproate? Pharmacopsychiatry. 2010;43(5):198–200.
Chaudrey KH, Naser TB, Steinberg A, Avashia KD, Nouri-Kolouri M, Asadi S, Irshad Khan SI, Ihsan M. Thinking beyond the obvious: hepatotoxicity secondary to idiosyncratic depakote toxicity. Am J Ther. 2012;19(6):403–6.
van Egmond H, Degomme P, de Simpel H, Dierick AM, Roels H. A suspected case of late-onset sodium-valproate-induced hepatic failure. Neuropediatrics. 1987;18(2):96–8.
Dickinson RG, Bassett ML, Searle J, Tyrer JH, Eadie MJ. Valproate hepatotoxicity: a review and report of two instances in adults. Clin Exp Neurol. 1985;21:79–91.
Itoh S, Yamaba Y, Matsuo S, Saka M, Ichinoe A. Sodium valproate-induced liver injury. Am J Gastroenterol. 1982;77(11):875–9.
Gerstner T, Bauer MO, Longin E, Bell N, Koenig SA. Reversible hepatotoxicity, pancreatitis, coagulation disorder and simultaneous bone marrow suppression with valproate in a 2-year-old girl. Seizure. 2007;16(6):554–6.
Bostancioglu M, Oner N, Kucukugurluoglu Y, Kaya M, Aladag N, Celtik C, Karasalihoglu S. Does valproate therapy decrease the bone mineral density in one-year follow-up in children? Trakya Univ Med Fak Derg. 2009;26(1):24–8.
Ecevit C, Aydoğan A, Kavakli T, Altinöz S. Effect of carbamazepine and valproate on bone mineral density. Pediatr Neurol. 2004;31(4):279–82.
Oner N, Kaya M, Karasalihoğlu S, Karaca H, Celtik C, Tütüncüler F. Bone mineral metabolism changes in epileptic children receiving valproic acid. J Paediatr Child Health. 2004;40(8):470–3.
Rugino TA, Janvier YM, Baunach JM, Bilat CA. Hypoalbuminemia with valproic acid administration. Pediatr Neurol. 2003;29(5):440–4.
Verrotti A, Agostinelli S, Coppola G, Parisi P, Chiarelli F. A 12-month longitudinal study of calcium metabolism and bone turnover during valproate monotherapy. Eur J Neurol. 2010;17(2):232–7.
Zare M, Ghazvini MR, Dashti M, Najafi MR, Alavi-Naeini AM. Bone turnover markers in epileptic patients under chronic valproate therapy. J Res Med Sci. 2013;18(4):338–40.
Caksen H, Dülger H, Cesur Y, Odabaş D, Tuncer O, Ataş B. No effect of long-term valproate therapy on thyroid and parathyroid functions in children. Int J Neurosci. 2002;112(11):1371–4.
Triantafyllou N, Lambrinoudaki I, Armeni E, Evangelopoulos EM, Boufidou F, Antoniou A, Tsivgoulis G. Effect of long-term valproate monotherapy on bone mineral density in adults with epilepsy. J Neurol Sci. 2010;290(1–2):131–4.
Bavbek N, Alkan R, Uz E, Kaftan O, Akcay A. Hyponatremia associated with sodium valproate in a 22-year-old male. Nephrol Dial Transplant. 2008;23(1):410.
Beers E, van Puijenbroek EP, Bartelink IH, van der Linden CM, Jansen PA. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) or hyponatraemia associated with valproic acid: four case reports from the Netherlands and a case/non-case analysis of vigibase. Drug Saf. 2010;33(1):47–55.
Branten AJ, Wetzels JF, Weber AM, Koene RA. Hyponatremia due to sodium valproate. Ann Neurol. 1998;43(2):265–7.
Kuo YC, Lin YC, Chen PH. Emerging hyperkalemia following valproic acid use in an elderly patient with late-onset mania. J Clin Psychopharmacol. 2016;36(4):394–5.
Miyaoka T, Seno H, Itoga M, Kishi T, Ishino H, Horiguchi J. Contribution of sodium valproate to the syndrome of inappropriate secretion of antidiuretic hormone. Int Clin Psychopharmacol. 2001;16(1):59–61.
Patel KR, Meesala A, Stanilla JK. Sodium valproate-induced hyponatremia: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(5):e1.
Conley EL, Coley KC, Pollock BG, Dapos SV, Maxwell R, Branch RA. Prevalence and risk of thrombocytopenia with valproic acid: experience at a psychiatric teaching hospital. Pharmacotherapy. 2001;21(11):1325–30.
May RB, Sunder TR. Hematologic manifestations of long-term valproate therapy. Epilepsia. 1993;34(6):1098–101.
Nasrullah M, Saleem K, Zaheer M, Bhatty AF, Shafiq F. Thrombocytopenia in epileptic patients on valproic acid. PJMHS. 2015;9(1):24–5.
Trannel TJ, Ahmed I, Goebert D. Occurrence of thrombocytopenia in psychiatric patients taking valproate. Am J Psychiatry. 2001;158(1):128–30.
Delgado MR, Riela AR, Mills J, Browne R, Roach ES. Thrombocytopenia secondary to high valproate levels in children with epilepsy. J Child Neurol. 1994;9(3):311–4.
Serdaroglu G, Tütüncüoglu S, Kavakli K, Tekgül H. Coagulation abnormalities and acquired von Willebrand’s disease type 1 in children receiving valproic acid. J Child Neurol. 2002;17(1):41–3.
Vasudev K, Keown P, Gibb I, McAllister-Williams RH. Hematological effects of valproate in psychiatric patients: what are the risk factors? J Clin Psychopharmacol. 2010;30(3):282–5.
Allarakhia IN, Garofalo EA, Komarynski MA, Robertson PL. Valproic acid and thrombocytopenia in children: a case-controlled retrospective study. Pediatr Neurol. 1996;14(4):303–7.
De Berardis D, Campanella D, Matera V, Gambi F, La Rovere R, Sepede G, Grimaldi MR, Pacilli AM, Salerno RM, Ferro FM. Thrombocytopenia during valproic acid treatment in young patients with new-onset bipolar disorder. J Clin Psychopharmacol. 2003;23(5):451–8.
Tariot PN, Schneider LS, Mintzer JE, Cutler AJ, Cunningham MR, Thomas JW, Sommerville KW. Safety and tolerability of divalproex sodium in the treatment of signs and symptoms of mania in elderly patients with dementia: Results of a double-blind, placebo-controlled trial. Curr Ther Res. 2001;62:51–67.
Attilakos A, Katsarou E, Voudris K, Garoufi A. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia. 2007;48(1):205–6.
Zeller JA, Schlesinger S, Runge U, Kessler C. Influence of valproate monotherapy on platelet activation and hematologic values. Epilepsia. 1999;40(2):186–9.
Gidal B, Spencer N, Maly M, Pitterle M, Williams E, Collins M, Jones J. Valproate-mediated disturbances of hemostasis: relationship to dose and plasma concentration. Neurology. 1994;44(8):1418–22.
Ko CH, Kong CK, Tse PW. Valproic acid and thrombocytopenia: cross-sectional study. Hong Kong Med J. 2001;7(1):15–21.
Koenig S, Gerstner T, Keller A, Teich M, Longin E, Dempfle CE. High incidence of vaproate-induced coagulation disorders in children receiving valproic acid: a prospective study. Blood Coagul Fibrinolysis. 2008;19(5):375–82.
Nasreddine W, Beydoun A. Valproate-induced thrombocytopenia: a prospective monotherapy study. Epilepsia. 2008;49(3):438–45.
Verrotti A, Greco R, Matera V, Altobelli E, Morgese G, Chiarelli F. Platelet count and function in children receiving sodium valproate. Pediatr Neurol. 1999;21(3):611–4.
Tohen M, Castillo J, Baldessarini RJ, Zarate C Jr, Kando JC. Blood dyscrasias with carbamazepine and valproate: a pharmacoepidemiological study of 2,228 patients at risk. Am J Psychiatry. 1995;152(3):413–8.
Rahman A, Mican LM, Fischer C, Campbell AH. Evaluating the incidence of leukopenia and neutropenia with valproate, quetiapine, or the combination in children and adolescents. Ann Pharmacother. 2009;43(5):822–30.
Park HJ, Kim JY. Incidence of neutropenia with valproate and quetiapine combination treatment in subjects with acquired brain injuries. Arch Phys Med Rehabil. 2016;97(2):183–8.
Taymur I, Sarı S, Güngör B, İnel A, Dağlı O. A case of thrombocytopenia and anemia due to use of valproic acid. J Mood Disord. 2015;5(2):88–9.
Nerumalla CS, Shah AA. A case of thrombocytopenia associated with valproic acid treatment. Prim Care Companion CNS Disord. 2013;. https://doi.org/10.4088/PCC.13l01526.
Tseng YT, Ho PS, Wang CF, Liang CS. Valproic acid-induced thrombocytopenia may cause wound nonhealing in individuals with schizophrenia. Psychosomatics. 2015;56(4):410–3.
Fawcett RG. Dose-related thrombocytopenia and macrocytic anemia associated with valproate use in bipolar disorder. J Clin Psychiatry. 1997;58(3):125.
Hoffman LM. Sodium valproate and thrombocytopenia. Can Med Assoc J. 1982;126(4):358–9.
Allan RW. Myelodysplastic syndrome associated with chronic valproic acid therapy: a case report and review of the literature. Hematology. 2007;12(6):493–6.
Storch DD. Severe leukopenia with valproate. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208–9.
Singh NK, Nagendra S. Reversible neutrophil abnormalities related to supratherapeutic valproic acid levels. Mayo Clin Proc. 2008;83(5):600.
Kohli U, Gulati S. Valproate induced isolated neutropenia. Indian J Pediatr. 2006;73(9):844.
Yurdaisik G, Uysal S. Valproic acid-induced neutropenia. Omu tip Dergi. 2002;19(2):130–2.
Symon DN, Russell G. Sodium valproate and neutropenia. Arch Dis Child. 1983;58(3):235.
Vesta KS, Medina PJ. Valproic acid-induced neutropenia. Ann Pharmacother. 2003;37(6):819–21.
Chakraborty S, Chakraborty J, Mandal S, Ghosal MK. A rare occurrence of isolated neutropenia with valproic acid: a case report. J Indian Med Assoc. 2011;109(5):345–6.
Stoner SC, Deal E, Lurk JT. Delayed-onset neutropenia with divalproex sodium. Ann Pharmacother. 2008;42(10):1507–10.
Chae BJ, Kang BJ. A case of delirium and subsequent pancytopenia associated with the oral loading of valproic acid. J Clin Psychiatry. 2005;66(6):801–2.
Kaya IS, Dilmen U, Toppare M, Senses DA, Prentice HG. Valproic-acid-induced pancytopenia and Coombs test positivity. Lancet. 1991;337(8751):1227–8.
Robinson D, Langer A, Casso D, Fenn H, Yesavage J. Pancytopenia and valproic acid–a possible association. J Am Geriatr Soc. 1995;43(2):198.
Stewart JT. Successful reintroduction of valproic acid after the occurrence of pancytopenia. Am J Geriatr Pharmacother. 2011;9(5):351–3.
Oluboka OJ, Haslam D, Gardner DM. Pancytopenia and valproic acid: a dose-related association. J Am Geriatr Soc. 2000;48(3):349–50.
Godaert L, Saint-Albin LA, Bousquet L, Fanon J-L, Drame M. Macrocytic isolated anaemia as an unusual adverse effect of divalproex sodium in the elderly. Eur Geriatr Med. 2016;7(5):403–4.
Kaczorowska-Hac B, Matheisel A, Maciejka-Kapuscinska L, Wisniewski J, Alska A, Adamkiewicz-Drozynska E, Balcerska A, Reszczynska I. Anemia secondary to valproic acid therapy in a 13-year-old boy: a case report. J Med Case Rep. 2012;10(6):239.
Kuo SC, Yeh YW, Chen CY, Yeh CB, Tzeng NS, Mao WC. Repeated adverse hematologic reactions associated with valproic acid use in a patient with acquired renal insufficiency. J Neuropsychiatry Clin Neurosci. 2013;25(1):E57–8 (Winter).
Ganick DJ, Sunder T, Finley JL. Severe hematologic toxicity of valproic acid. A report of four patients. Am J Pediatr Hematol Oncol. 1990;12(1):80–5 (Spring).
Gerstner T, Teich M, Bell N, Longin E, Dempfle CE, Brand J, König S. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia. 2006;47(7):1136–43.
Sleiman C, Raffy O, Roué C, Mal H. Fatal pulmonary hemorrhage during high-dose valproate monotherapy. Chest. 2000;117(2):613.
Hsu H-C, Tseng H-K, Wang S-C, Wang Y-Y. Valproic acid-induced agranulocytosis. Int J Gerontol. 2009;3(2):137–9.
Bottom KS, Adams DM, Mann KP, Ware RE. Trilineage hematopoietic toxicity associated with valproic acid therapy. J Pediatr Hematol Oncol. 1997;19(1):73–6.
Farkas V, Szabó M, Rényi I, Kohlhéb O, Benninger C. Temporary pure red-cell aplasia during valproate monotherapy: clinical observations and spectral electroencephalographic aspects. J Child Neurol. 2000;15(7):485–7.
The T, Kolla R, Dawkins F, Trouth AJ. Pure red cell aplasia after 13 years of sodium valproate, and bone marrow suppression after 17 years of carbamazepine. PLoS Med. 2004;1(2):e51.
Ahmed SN, Siddiqi ZA. Antiepileptic drugs and liver disease. Seizure. 2006;15(3):156–64.
Scheffner D, König S, Rauterberg-Ruland I, et al. Fatal liver failure in 16 children with valproate therapy. Epilepsia. 1988;29(5):530–42.
Vancampfort D, Vansteelandt K, Correll CU, Mitchell AJ, De Herdt A, Sienaert P, Probst M, De Hert M. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170(3):265–74.
Cooper SJ, Reynolds GP. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. 2016;30(8):717–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Rosanne W. Meijboom and Koen P. Grootens have no conflicts of interest to declare.
Funding
No funding was received for this study.
Rights and permissions
About this article
Cite this article
Meijboom, R.W., Grootens, K.P. Dispensability of Annual Laboratory Follow-Up After More than 2 Years of Valproic Acid Use: A Systematic Review. CNS Drugs 31, 939–957 (2017). https://doi.org/10.1007/s40263-017-0479-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-017-0479-z