Abstract
Background
Lactating mothers taking ezetimibe, an antihyperlipidemic agent, may be hesitant to breastfeed despite the known benefit of breastfeeding to both mother and infant. Currently, no data exist on the presence or concentration of ezetimibe and its main active metabolite, ezetimibe-glucuronide (EZE-glucuronide), in human breast milk.
Methods
Voluntary breast milk samples containing ezetimibe and EZE-glucuronide were attained from lactating mothers taking ezetimibe as part of their treatment. An assay was developed and validated to measure ezetimibe and EZE-glucuronide concentrations in breast milk. A workflow that utilized a developed and evaluated pediatric physiologically based pharmacokinetic (PBPK) model, the measured concentrations in milk, and weight-normalized breast milk intake volumes was applied to predict infant exposures and determine the upper area under the curve ratio (UAR).
Results
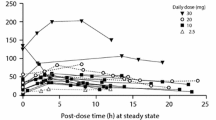
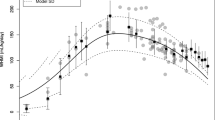
Fifteen breast milk samples from two maternal-infant pairs were collected. The developed liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay showed an analytical range of 0.039–5.0 ng/mL and 0.39–50.0 ng/mL for ezetimibe and EZE-glucuronide, respectively. The measured concentrations in the breast milk samples were 0.17–1.02 ng/mL and 0.42–2.65 ng/mL of ezetimibe and EZE-glucuronide, respectively. The evaluated pediatric PBPK model demonstrated minimal exposure overlap in adult therapeutic dose and breastfed infant simulated area under the concentration-time curve from time zero to 24 h (AUC24). Calculated UAR across infant age groups ranged from 0.0015 to 0.0026.
Conclusions
PBPK model-predicted ezetimibe and EZE-glucuronide exposures and UAR suggest that breastfeeding infants would receive non-therapeutic exposures. Future work should involve a ‘mother-infant pair study’ to ascertain breastfed infant plasma ezetimibe and EZE-glucuronide concentrations to confirm the findings of this work.




Similar content being viewed by others
References
Godfrey JR, Lawrence RA. Toward optimal health: the maternal benefits of breastfeeding. J Women’s Health (2002). 2010;19(9):1597–602.
Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):96–113.
Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess. 2007;153:1–186.
Ip S, Chung M, Raman G, Trikalinos TA, Lau J. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4(Suppl 1):S17-30.
Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117(2):425–32.
Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–91.
Mazer-Amirshahi M, Samiee-Zafarghandy S, Gray G, van den Anker JN. Trends in pregnancy labeling and data quality for US-approved pharmaceuticals. Am J Obstet Gynecol. 2014;211(6):690.e1-11.
Jones H, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacomet Syst Pharmacol. 2013;2(8): e63.
Yeung CHT, Ito S, Autmizguine J, Edginton AN. Incorporating breastfeeding-related variability with physiologically based pharmacokinetic modeling to predict infant exposure to maternal medication through breast milk: A workflow applied to lamotrigine. AAPS J. 2021;23(4):70.
Yeung CHT, Fong S, Malik PRV, Edginton AN. Quantifying breast milk intake by term and preterm infants for input into paediatric physiologically based pharmacokinetic models. Matern Child Nutr. 2020;16(2): e12938.
Bennett PN, Notrianni LJ. Risk from drugs in breast milk: an analysis by relative dose. Br J Clin Pharmacol. 1996;42:623–4.
National Institutes of Health Clinical Center (CC). Higher-Dose Ezetimibe to Treat Homozygous Sitosterolemia. 2008 [cited 6 Feb 2023]. https://clinicaltrials.gov/ct2/show/NCT00099996.
European Medicines Agency. Ezetimibe tablet 10 mg product-specific bioequivalence guidance. European Medicines Agency; 2018.
Kosoglou T, Maxwell S, Chung C, Batra V, Statkevich P. Dose-proportionality of ezetimibe. Clin Pharmacol Ther. 2002;71(2):P97.
Pharmacutical and Food Safety Bureau. Report on the Deliberation Results-Zetia Tablets 10 mg. Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour, and Welfare; 2007.
Schering-Plough. Review Report-Zetia Tablets 10 mg. Pharmaceutical and Medical Devices Agency; 2003.
Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283(1):157–63.
Patrick JE, Kosoglou T, Stauber KL, Alton KB, Maxwell SE, Zhu Y, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos. 2002;30(4):430–7.
Oswald S, Haenisch S, Fricke C, Sudhop T, Remmler C, Giessmann T, et al. Intestinal expression of P-glycoprotein (ABCB1), multidrug resistance associated protein 2 (ABCC2), and uridine diphosphate-glucuronosyltransferase 1A1 predicts the disposition and modulates the effects of the cholesterol absorption inhibitor ezetimibe in humans. Clin Pharmacol Ther. 2006;79(3):206–17.
Oswald S, König J, Lütjohann D, Giessmann T, Kroemer HK, Rimmbach C, et al. Disposition of ezetimibe is influenced by polymorphisms of the hepatic uptake carrier OATP1B1. Pharmacogenet Genomics. 2008;18(7):559–68.
de Waart DR, Vlaming ML, Kunne C, Schinkel AH, Oude Elferink RP. Complex pharmacokinetic behavior of ezetimibe depends on abcc2, abcc3, and abcg2. Drug Metab Dispos Biol Fate Chem. 2009;37(8):1698–702.
MSP Singapore Co. Clinical pharmacology and biopharmaceutics review(s)—ZETIA. Center for Drug Evaluation and Research; 2001.
Sanis Health Inc. Ezetimibe Tablets Package Insert Product Monograph. Sanis Health Inc.; 2014.
Drugs and Lactation Database (LactMed®): Ezetimibe. 19 Oct 2020.
Guo L, Wang MM, He M, Qiu FR, Jiang J. Simultaneous determination of ezetimibe and its glucuronide metabolite in human plasma by solid phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2015;986–987:108–14.
Broughton PM. Carry-over in automatic analysers. J Autom Chem. 1984;6(2):94–5.
Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15(2):455–64.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Rodgers T, Rowland M. Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res. 2007;24(5):918–33.
Schmitt W. General approach for the calculation of tissue to plasma partition coefficients. Toxicol In Vitro. 2008;22(2):457–67.
Ghosal A, Hapangama N, Yuan Y, Achanfuo-Yeboah J, Iannucci R, Chowdhury S, et al. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia). Drug Metab Dispos. 2004;32(3):314–20.
Shiran MR, Proctor NJ, Howgate EM, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. Prediction of metabolic drug clearance in humans: in vitro-in vivo extrapolation vs allometric scaling. Xenobiotica. 2006;36(7):567–80.
Rostami-Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6(2):140–8.
Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20(6):452–77.
Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet. 2006;21(5):357–74.
Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2003;123(5):369–75.
Achour B, Dantonio A, Niosi M, Novak JJ, Fallon JK, Barber J, et al. Quantitative characterization of major hepatic UDP-glucuronosyltransferase enzymes in human liver microsomes: comparison of two proteomic methods and correlation with catalytic activity. Drug Metab Dispos. 2017;45(10):1102–12.
Prasad B, Johnson K, Billington S, Lee C, Chung GW, Brown CD, et al. Abundance of drug transporters in the human kidney cortex as quantified by quantitative targeted proteomics. Drug Metab Dispos. 2016;44(12):1920–4.
Food and Drug Administration. Guidance for industry—dissolution testing of immediate release solid oral dosage forms. Center for Drug Evaluation and Research; 1997.
Burt HJ, Riedmaier AE, Harwood MD, Crewe HK, Gill KL, Neuhoff S. Abundance of hepatic transporters in caucasians: a meta-analysis. Drug Metab Dispos. 2016;44(10):1550–61.
Willmann S, Höhn K, Edginton A, Sevestre M, Solodenko J, Weiss W, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34(3):401–31.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45(10):1013–34.
Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45(7):683–704.
Divakaran K, Hines RN, McCarver DG. Human hepatic UGT2B15 developmental expression. Toxicol Sci. 2014;141(1):292–9.
Badée J, Qiu N, Collier AC, Takahashi RH, Forrest WF, Parrott N, et al. Characterization of the ontogeny of hepatic UDP-glucuronosyltransferase enzymes based on glucuronidation activity measured in human liver microsomes. J Clin Pharmacol. 2019;59(Suppl 1):S42–55.
Prasad B, Gaedigk A, Vrana M, Gaedigk R, Leeder JS, Salphati L, et al. Ontogeny of hepatic drug transporters as quantified by LC-MS/MS proteomics. Clin Pharmacol Ther. 2016;100(4):362–70.
Kusters DM, Caceres M, Coll M, Cuffie C, Gagné C, Jacobson MS, et al. Efficacy and safety of ezetimibe monotherapy in children with heterozygous familial or nonfamilial hypercholesterolemia. J Pediatrics. 2015;166(6):1377-84.e1-3.
Burt VL, Harris T. The third National Health and Nutrition Examination Survey: contributing data on aging and health. Gerontologist. 1994;34(4):486–90.
Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: NHANES III. [cited 17 Jan 2023]. Available at: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx
Yeung CHT, Houle SKD, Anderson PO, Best BM, Dubinsky S, Edginton AN. Addressing maternal medication use during breastfeeding using clinical resources and a novel physiologically based pharmacokinetic model-derived metric: A qualitative study. Front Pediatr. 2023;11:1147566.
Saito J, Kaneko K, Abe S, Yakuwa N, Kawasaki H, Suzuki T, et al. Pravastatin concentrations in maternal serum, umbilical cord serum, breast milk and neonatal serum during pregnancy and lactation: A case study. J Clin Pharm Ther. 2022;47(5):703–6.
Yang H, Zhang D, Mei S, Zhao Z. Simultaneous determination of plasma lamotrigine, lamotrigine N2-glucuronide and lamotrigine N2-oxide by UHPLC-MS/MS in epileptic patients. J Pharm Biomed Anal. 2022;220: 115017.
Cooper JD, Shearsby NJ, Taylor JE, Fook Sheung CT. Simultaneous determination of lamotrigine and its glucuronide and methylated metabolites in human plasma by automated sequential trace enrichment of dialysates and gradient high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;702(1–2):227–33.
Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44(5):467–94.
Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Clinical lactation studies: considerations for study design. Rockville: US Department of Health and Human Services, Food and Drug Administration; 2019.
Reyderman L, Kosoglou T, Statkevich L, Pemper L, Maxwell S, Batra V. Pharmacokinetics of ezetimibe in subjects with normal renal function or severe chronic renal insufficiency. Clin Pharmacol Ther. 2002;71(2):P27.
US FDA. Guidance for industry—dissolution testing of immediate release solid oral dosage forms. Center for Drug Evaluation and Research; 1997.
Bae J-W, Choi C-I, Park S-H, Jang C-G, Lee S-Y. Analytical LC-MS/MS method for ezetimibe and its application for pharmacokinetic study. J Liq Chromatogr Relat Technol. 2012;35(1):141–52.
Bergman AJ, Burke J, Larson P, Johnson-Levonas AO, Reyderman L, Statkevich P, et al. Interaction of single-dose ezetimibe and steady-state cyclosporine in renal transplant patients. J Clin Pharmacol. 2006;46(3):328–36.
Gustavson LE, Schweitzer SM, Burt DA, Achari R, Rieser MJ, Edeki T, et al. Evaluation of the potential for pharmacokinetic interaction between fenofibrate and ezetimibe: a phase I, open-label, multiple-dose, three-period crossover study in healthy subjects. Clin Ther. 2006;28(3):373–87.
Jackson A, D’Avolio A, Watson V, Bonora S, Back D, Taylor J, et al. Pharmacokinetics and safety of the co-administration of the antiretroviral raltegravir and the lipid-lowering drug ezetimibe in healthy volunteers. J Antimicrob Chemother. 2011;66(4):885–9.
Kim CH, An H, Kim SH, Shin D. Pharmacokinetic and pharmacodynamic interaction between ezetimibe and rosuvastatin in healthy male subjects. Drug Des Dev Ther. 2017;11:3461–9.
Kim H, Choi HY, Kim YH, Bae KS, Jung J, Son H, et al. Pharmacokinetic interactions and tolerability of rosuvastatin and ezetimibe: an open-label, randomized, multiple-dose, crossover study in healthy male volunteers. Drug Des Dev Ther. 2018;12:815–21.
Kosoglou T, Statkevich P, Fruchart JC, Pember LJ, Reyderman L, Cutler DL, et al. Pharmacodynamic and pharmacokinetic interaction between fenofibrate and ezetimibe. Curr Med Res Opin. 2004;20(8):1197–207.
Kosoglou T, Statkevich P, Yang B, Suresh R, Zhu Y, Boutros T, et al. Pharmacodynamic interaction between ezetimibe and rosuvastatin. Curr Med Res Opin. 2004;20(8):1185–95.
Oswald S, Meyer zu Schwabedissen HE, Nassif A, Modess C, Desta Z, Ogburn ET, et al. Impact of efavirenz on intestinal metabolism and transport: insights from an interaction study with ezetimibe in healthy volunteers. Clin Pharmacol Therap. 2012;91(3):506–13.
Reyderman L, Kosoglou T, Boutros T, Seiberling M, Statkevich P. Pharmacokinetic interaction between ezetimibe and lovastatin in healthy volunteers. Curr Med Res Opin. 2004;20(9):1493–500.
Reyderman L, Kosoglou T, Statkevich P, Pember L, Boutros T, Maxwell SE, et al. Assessment of a multiple-dose drug interaction between ezetimibe, a novel selective cholesterol absorption inhibitor and gemfibrozil. Int J Clin Pharmacol Ther. 2004;42(9):512–8.
Reyderman L, Kosoglou T, Cutler DL, Maxwell S, Statkevich P. The effect of fluvastatin on the pharmacokinetics and pharmacodynamics of ezetimibe. Curr Med Res Opin. 2005;21(8):1171–9.
Acknowledgments
The authors would like to acknowledge Medela Canada Inc. for providing electric breast pumps for this study. They would also like to acknowledge the Lipid Clinic at St. Boniface Hospital for their contributions in arranging the collection of breast milk samples and storage, and completing patient questionnaires. This work was based on Cindy Hoi Ting Yeung’s thesis presented to the University of Waterloo in partial fulfilment of the requirement for the degree of Doctor of Philosophy in Pharmacy. A copy of the thesis is available from the University of Waterloo at https://libuwspaceprd02.uwaterloo.ca/bitstream/handle/10012/19412/Yeung_CindyHoiTing.pdf?isAllowed=y&sequence=7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was funded by the Canadian Institutes of Health Research (CIHR) Project Grant PJT-159782, and CIHR Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award (CGS-D), a Canada Scholarship to Honour Nelson Mandela, DF2-171445.
Conflicts of Interest
Cindy H.T. Yeung, Julie Autmizguine, Pooja Dalvi, Audrey Denoncourt, Shinya Ito, Pamela Katz, Mehzabin Rahman, Yves Theoret, and Andrea N. Edginton have no conflicts of interest to disclose relevant to this article.
Availability of Data and Material
A de-identified dataset can be shared with the appropriate approval. The requestor would need to contact the Principal Investigator (Dr. Shinya Ito, shinya.ito@sickkids.ca) with the request.
Ethics Approval
This study received ethics clearance from the REBs of the University of Manitoba (#HS19991) and the University of Waterloo (REB # 41155).
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Code Availability
Not applicable.
Author Contributions
CHTY, SI, and ANE conceptualized and designed the study. JA, AD, and YT developed the assay. PD, PK, and MR recruited patients and performed data collection. CHTY wrote the original draft manuscript. All authors reviewed and revised the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeung, C.H.T., Autmizguine, J., Dalvi, P. et al. Maternal Ezetimibe Concentrations Measured in Breast Milk and Its Use in Breastfeeding Infant Exposure Predictions. Clin Pharmacokinet 63, 317–332 (2024). https://doi.org/10.1007/s40262-023-01345-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01345-0