Abstract
Background and Objectives
Nasal esketamine is indicated for the treatment of adults with treatment-resistant depression and depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Primary objectives of this study were to evaluate the effect of nasal decongestant pretreatment in patients with allergic rhinitis and the impact of daily nasal corticosteroid administration by healthy subjects on nasal esketamine pharmacokinetics.
Methods
Patients with allergic rhinitis self-administered 56 mg of nasal esketamine after pretreatment with nasal oxymetazoline (0.05%) at 1 h before esketamine and without oxymetazoline pretreatment. They were exposed to grass pollen in an allergen challenge chamber to induce allergic rhinitis symptoms at approximately 2 h before each esketamine administration until 1 h after. Healthy subjects self-administered esketamine (56 mg) before and after administration for 16 consecutive days of mometasone (200 µg), with the second esketamine dose administered 1 h after the last mometasone dose. The plasma pharmacokinetics of esketamine and noresketamine were assessed after each esketamine administration. The tolerability of esketamine, including effects on dissociative and potential psychotomimetic symptoms and level of sedation and suicidal ideation and behavior, was evaluated.
Results
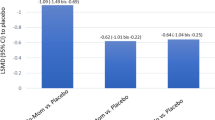
The rate of esketamine absorption was slightly greater in patients exhibiting symptoms of allergic rhinitis (decrease in median tmax from 32 min to 22 min). Increases in esketamine Cmax and AUC were also small (mean, ≤ 21%). The pharmacokinetics of esketamine was not affected by oxymetazoline or mometasone pretreatment. Esketamine was well tolerated when it was administered with or without pretreatment of oxymetazoline or mometasone.
Conclusions
Patients exhibiting symptoms of rhinitis may receive nasal esketamine spray without dose adjustment. In addition, esketamine may be administered 1 h after using a nasal decongestant or corticosteroid.
Trial Registration
The study was registered in the Clinical Trials (NCT02154334) and EudraCT (2014‐000534‐38) registries.




Similar content being viewed by others
Change history
05 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40262-023-01290-y
References
WHO Fact Sheet on Depression 13 September 2021; http://www.who.int/news-room/fact-sheets/detail/depression. Assessed 6 Mar 2023.
Machado-Vieira R, Salvadore G, Luckenbaugh D, et al. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depression. J Clin Psychiatry. 2008;69(6):946–58. https://doi.org/10.4088/jcp.v69n0610.
Rush A, Trivedi M, Wisniewski S, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905–17. https://doi.org/10.1176/ajp.2006.163.11.1905.
SPRAVATO (esketamine) nasal spray [package insert]. Janssen Pharmaceuticals Companies, Titusville, NJ; 2019. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SPRAVATO-pi.pdf. Accessed 16 June 2023.
SPRAVATO (esketamine) nasal spray EMA Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/spravato-epar-product-information_en.pdf. Accessed 16 June 2023.
Daly E, Singh J, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression. A randomized clinical trial. JAMA Psychiat. 2018;75(2):139–48. https://doi.org/10.1001/jamapsychiatry.2017.3739.
Zanos P, Moaddel R, Morris P, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–60. https://doi.org/10.1124/pr.117.015198.
Duman R, Aghajanian G, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–49. https://doi.org/10.1038/nm.4050.
Manji H, Drevets W, Charney D. The cellular neurobiology of depression. Nat Med. 2001;7(5):541–7. https://doi.org/10.1038/87865.
Murrough J, Abdallah C, Mathew S. Targeting glutamate signaling in depression: progress and prospects. Nat Rev Drug Discov. 2017;16(7):472–86. https://doi.org/10.1038/nrd.2017.16.
Illum L. Nasal drug delivery-possibilities, problems and solutions. J Controlled Release. 2003;87(1–3):187–98. https://doi.org/10.1016/s0168-3659(02)00363-2.
Türker S, Onure E, Özer Y. Nasal and drug delivery systems. Pharm World Sci. 2004;26(3):137–42. https://doi.org/10.1023/b:phar.0000026823.82950.ff.
Perez-Ruixo C, Rossenu S, Zannikos P, et al. Population pharmacokinetics of esketamine nasal spray and its metabolite noresketamine in healthy subjects and patients with treatment-resistant depression. Clin Pharmacokinetics. 2021;60(4):501–16. https://doi.org/10.1007/s40262-020-00953-4.
Portmann S, Kwan H, Theurillat R, et al. Enantioselective capillary electrophoresis for identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J Chromatogr A. 2010;1217(51):7942–8. https://doi.org/10.1016/j.chroma.2010.06.028.
Yanagihara Y, Kariya S, Ohtani M et al. Involvement of CYP2B6 in N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2001;29(6):887-890. https://dmd.aspetjournals.org/content/29/6/887.long. Accessed 16 June 2023.
www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/in-depth-review-of-allergic-rhinitis. Assessed 6 Mar 2023.
Krug N, Loedding H, Hohlfeld J, et al. Validation of an environmental exposure unit for controlled human inhalation studies with grass pollen in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2003;33(12):1667–74. https://doi.org/10.1111/j.1365-2222.2003.01810.x.
Hohlfeld JM, Holland-Letz T, Larbig M, et al. Diagnostic value of outcome measures following allergen exposure in an environmental challenge chamber compared with natural conditions. Clin Exp Allergy. 2010;40(7):998–1006. https://doi.org/10.1111/j.1365-2222.2010.03498.x.
Badorrek P, Dick M, Hecker H, et al. Anti-allergic drug testing in an environmental challenge chamber is suitable both in and out of the relevant pollen season. Ann Allergy Asthma Immunol. 2011;106(4):336–41. https://doi.org/10.1016/j.anai.2010.12.018.
Eberta B, Mikkelsen S, Thorkildsen C, et al. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333(1):99–104. https://doi.org/10.1016/s0014-2999(97)01116-3.
Holtman J, Crooks P, Johnson-Hardy J, et al. Effects of norketamine enantiomers in rodent models of persistent pain. Pharmacol Biochem Behav. 2008;90(4):676–85. https://doi.org/10.1016/j.pbb.2008.05.011.
Swartjes M, Morariu A, Niesters M, et al. Nonselective and NR2B-selective N-methyl-D-aspartic acid receptor antagonists produce antinociception and long-term relief of allodynia in acute and neuropathic pain. Anesthesiology. 2011;115(1):165–74. https://doi.org/10.1097/ALN.0b013e31821bdb9b.
Moaddel R. Sub-anesthetic concentrations of (R, S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698(1–3):228–34. https://doi.org/10.1016/j.ejphar.2012.11.023.
Geisslinger G, Hering W, Thomann P, et al. Pharmacokinetics and pharmacodynamics of ketamine enantiomers in surgical patients using a stereoselective analytical method. Br J Anaesth. 1993;70(6):666–71. https://doi.org/10.1093/bja/70.6.666.
Ihmsen H, Geisslinger G, Schuttler J. Sterioselective pharmacokinetics of ketamine: R(-)-ketamine inhibits the elimination of S(+)-ketamine. Clin Pharmacol Ther. 2001;70(5):431–8. https://doi.org/10.1016/S0009-9236(01)06321-4.
Hartvig P. Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther. 1995;58(2):165–73. https://doi.org/10.1016/0009-9236(95)90194-9.
Bremner J, Krystal J, Putnam F, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1998;11(1):125–36. https://doi.org/10.1023/A:1024465317902.
Pambianco D, Vargo J, Pruitt R, et al. Computer-assisted personalized sedation for upper endoscopy and colonoscopy: a comparative, multicenter randomized study. Gastrointest Endosc. 2011;73(4):765–72. https://doi.org/10.1016/j.gie.2010.10.031.
Posner K, Oquendo M, Gould M, et al. Columbia classification algorithm of suicide assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164(7):1035–43. https://doi.org/10.1176/appi.ajp.164.7.1035.
Seidman M, Guideline Otolaryngology Development Group. AAO-HNSF. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(S1):S1–43. https://doi.org/10.1177/0194599814561600.
Dykewicz M, Hamilos D. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125(2):S103–15. https://doi.org/10.1016/j.jaci.2009.12.989.
Perelman M, Fisher A, Smith A, et al. Impact of allergic rhinitis and its treatment on the pharmacokinetics of nasally administered fentanyl. Int J Clin Pharmacol Ther. 2013;51(5):349–56. https://doi.org/10.5414/CP201825.
Nave R, Sides E, Colberg T, et al. Pharmacokinetics of intranasal fentanyl spray (INFS) in subjects with seasonal allergic rhinitis with and without prior administration of oxymetzoline. Eur J Pain. 2009;13(S1):S55–285. https://doi.org/10.1016/S1090-3801(09)60721-7.
Davis G, Rudy A, Archer S, et al. Bioavailability and pharmacokinetics of intranasal hydromorphone in patients experiencing vasomotor rhinitis. Clin Drug Invest. 2004;24(11):633–9. https://doi.org/10.2165/00044011-200424110-00002.
Wood C, Fireman P, Grossman J, et al. Product characteristics and pharmacokinetics of intranasal ipratropium bromide. J Allergy Clin Immunol. 1995;95(5 Pt 2):1111–6. https://doi.org/10.1016/s0091-6749(95)70214-8.
Argenti D, Colligon I, Heald D, et al. Nasal mucosal inflammation has no effect on the absorption of intranasal triamcinolone acetonide. J Clin Pharmacol. 1994;34(8):854–8. https://doi.org/10.1002/j.1552-4604.1994.tb02051.x.
Shyu W, Pittman K, Robinson D, et al. The absolute bioavailability of transnasal butorphanol in patients experiencing rhinitis. Eur J Clin Pharmacol. 1993;45(6):559–62. https://doi.org/10.1007/BF00315315.
Lunell E, Molander L, Andersson M. Relative bioavailability of nicotine from a nasal spray in infectious rhinitis and after use of a topical decongestant. Eur J Clin Pharmacol. 1995;48(1):71–5. https://doi.org/10.1007/BF00202176.
Klimek L, Sperl A, Becker S, et al. Current therapeutical strategies for allergic rhinitis. Expert Opin Pharmacother. 2019;20(1):83–9. https://doi.org/10.1080/14656566.2018.1543401.
Williams D. Clinical pharmacology of corticosteroids. Respir Care. 2018;63(6):655–70. https://doi.org/10.4187/respcare.06314.
Wallace D. The diagnosis and management of rhinitis: An updated practice parameter. J Allergy Clin Immunol. 2008;122(2 Suppl):S1–84. https://doi.org/10.1016/j.jaci.2008.06.003.
Singh JB, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424–31. https://doi.org/10.1016/j.biopsych.2015.10.018.
Singh J, Fedgchin M, Daly E, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816–26. https://doi.org/10.1176/appi.ajp.2016.16010037.
Murrough J, Iosifescu D, Chang L, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–42. https://doi.org/10.1176/appi.ajp.2013.13030392.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The clinical study and the analyses presented were supported by research funding from Janssen Research and Development, LLC.
Conflict of Interest
Peter Zannikos, Bhavna Solanki, Marc De Meulder, and Jaskaran Singh were employed by Janssen Research & Development, LLC at the time the study was conducted and, as such, may have been eligible for stock and stock options.
Data Availability and Material
The datasets generated and/or analyzed during the study belong to Janssen Research & Development, LLC and are not publicly available.
Ethics Approval
The protocol was approved by an Institutional Review Board (Medizinische Hochshule Hannover Carl-Neuberg-Strasse 1, 30625 Hannover, Germany). The study was conducted at a single center (Fraunhofer Institute for Toxicology and Experimental Medicine, Hannover, Germany) in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirements. The study was registered in the Clinical Trials (NCT02154334) and EudraCT (2014‐000534‐38) registries.
Consent to Participate
Written informed consent was obtained from each participant before enrollment in the study after being advised of the potential risks and benefits of the study, as well as the investigational nature of the study.
Consent for Publication
All authors provided final approval of the published version.
Code Availability
Not applicable.
Author Contribution
All authors participated in the study design and implementation and/or conduct of the study. All authors read and approved the final manuscript.
Additional information
The original online version of this article was revised to correct Table 1 footnote.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zannikos, P., Solanki, B., De Meulder, M. et al. Pharmacokinetics of Nasal Esketamine in Patients with Allergic Rhinitis with and Without Nasal Decongestant Pretreatment and in Healthy Subjects with and Without Nasal Corticosteroid Pretreatment. Clin Pharmacokinet 62, 1315–1328 (2023). https://doi.org/10.1007/s40262-023-01273-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01273-z