Abstract
Cardiovascular diseases are the leading cause of death worldwide. Although there have been substantial advances over the last decades, recurrent adverse cardiovascular events after myocardial infarction are still frequent, particularly during the first year of the index event. For decades, high-density lipoprotein (HDL) has been among the therapeutic targets for long-term prevention after an ischemic event. However, early trials focusing on increasing HDL circulating levels showed no improvement in clinical outcomes. Recently, the paradigm has shifted to increasing the functionality of HDL rather than its circulating plasma levels. For this purpose, apolipoprotein-AI-based infusion therapies have been developed, including reconstituted HDL, such as CSL112. During the last decade, CSL112 has been extensively studied in Phase 1 and 2 trials and has shown promising results. In particular, CSL112 has been studied in the Phase 2b AEGIS trial exhibiting good safety and tolerability profiles, which has led to the ongoing large-scale Phase 3 AEGIS-II trial. This systematic overview will provide a comprehensive summary of the CSL112 drug development program focusing on its pharmacodynamic, pharmacokinetic, and safety profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Post-myocardial infarction (MI) patients are at high risk of recurrent major adverse cardiac events (MACE), particularly 90 days after the index event. |
Early trials focused on increasing circulating levels of high-density lipoprotein (HDL) failed to show a clinical benefit. Therefore, the focus has shifted towards increasing HDL function as assessed by means of cholesterol efflux capacity. |
CSL112 is a reconstituted HDL that effectively enhances cholesterol efflux capacity with optimal safety, tolerability, and pharmacokinetic/pharmacodynamic profiles. |
AEGIS-I trial, a Phase 2b trial, showed that CSL112 was not associated with renal or hepatic toxicity in patients with a recent MI [i.e., within 7 days of the index event]. |
The ongoing large-scale Phase 3 AEGIS-II trial will evaluate the safety and efficacy of CSL112 for the prevention of recurrent MACE in over 18,000 high-risk post-MI patients at 90 days’ follow-up. |
1 Introduction
Over the last decades, we assisted in landmark medical advances in reducing the cardiovascular (CV) risk associated with therapeutics affecting the lipid, inflammatory, and thrombotic pathways [1]. Despite these advances, patients who have suffered from a myocardial infarction (MI) are still at high risk of recurrent CV events during the first year (10.9–18.9%), with most events occurring within the first 90 days [2,3,4,5]. The burden of recurrent adverse events (AEs) directly impact long-term patient prognosis with significant financial impact [6, 7]. Therefore, tackling recurrent CV events in post-MI patients represents an urgent unmet clinical need.
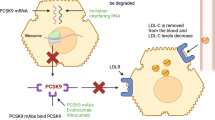
Among potential therapeutic targets to reduce recurrent CV events, it is the high-density lipoprotein cholesterol (HDL-C). High-density lipoprotein cholesterol is incorporated in high-density lipoprotein (HDL) particles, one of the five major groups of lipoproteins that perform an essential role in reverse cholesterol transport [8]. Since the early 1960s, a consistent inverse relation between HDL-C levels and coronary artery disease (CAD), the so-called “HDL hypothesis” has been described [9]. The quest for a better understanding of this association led to the discovery of the reverse cholesterol transport (RCT) pathway. Reverse cholesterol transport is the multistep process by which cholesterol is removed from cells in peripheral tissues (including lipid-laden macrophages in atherosclerotic plaques), enters the circulation, and is excreted in the feces (Fig. 1).
High-density lipoprotein (HDL) structure and metabolism. Simplified HDL physiology and CSL112 mechanism of action. Reverse cholesterol transfer (RCT) is a process mediated by HDL through which cholesterol is picked up from lipid-laden macrophages in peripheral cells/tissues and is delivered to the liver or intestine for its removal. Cholesterol efflux is the first step of RCT and consists of free-cholesterol (F-Ch) transfer from cells to HDL. Cholesterol efflux pathway can occur in different ways. A Via the interaction between ATP binding cassette A1 receptor (ABCA1) and ApoA-I (i.e., main component of HDL). This interaction mostly involves smaller subsets of HDL such as pre-β1-HDL (i.e., poor lipidated poA-I and discoid HDL). B Pre-β1-HDL mature into bigger and spherical HDL (i.e., α-HDL) by the action of the lecithin–cholesterol acyltransferase (LCAT), that transform F-Ch into esterified cholesterol (E-Ch). E-CH then migrates to the particle’s core while F-Ch maintains on the membrane. C ATP binding cassette G1 receptor (ABCG1), scavenger receptor class B type 1 (SR-BI) receptor, and passive cholesterol transport pathways are other cholesterol efflux modalities mostly employed by mature HDL. D E-Ch can be transferred from mature HDL by cholesteryl ester transferase protein (CETP) to ApoB lipoproteins in exchange for triglycerides (TG). E E-Ch delivery by mature HDL to the hepatic cells can occur either directly, via SR-BI receptor-mediated or indirectly, through hepatocytes- LDL-receptor mediated-uptake of ApoB containing lipoproteins (i.e., very-low-density lipoprotein [VDL], lipoprotein(a) [Lp(a)], intermediate-density lipoprotein [IDL], and low-density lipoprotein [LDL]). (4b) The liver can excrete F-Ch into the bile as F-Ch or bile salt. F In a pathway known as transintestinal cholesterol efflux (TICE), F-cholesterol can be directly transferred to enterocytes and ultimately poured into the intestinal lumen. PL phospholipid, rHDL reconstituted high-density lipoprotein (rHDL)
Several drugs have targeted different steps of the RCT pathway for raising HDL-C levels by either increasing its production (e.g., niacin) or decreasing its degradation (e.g., cholesteryl ester transfer protein [CETP] inhibitors). However, Phase 3 randomized trials failed to provide strong evidence to support the role of these drugs in clinical practice [10]. Among these, anacetrapib (a CETP inhibitor) was associated with a significant increase in HDL-C, reduced low-density lipoprotein cholesterol (LDL-C), and major adverse cardiovascular events (MACE) [11]. Nevertheless, since anacetrapib was associated with LDL-C reduction, it is unclear if the clinical benefit can be entirely associated with an increase in HDL-C. Overall, the most accepted explanation of why these therapies failed is that simply raising HDL concentrations does not translate into reducing MACE.
To date, there is evidence that increasing HDL's function may provide better outcomes than increasing its circulating levels [12]. Cholesterol efflux capacity (CEC) is an ex vivo methodology to assess HDL function, a predictor of CAD independent of HDL-C levels [13]. A high CEC is associated with a reduction of adverse CV events [14, 15]. Cholesterol efflux capacity impairment is frequently encountered in patients with MI, contributing to lipid accumulation, inflammation, and endothelial dysfunction [15, 16]. This paradigm change represents the shift from the “HDL hypothesis” to the “CEC hypothesis.”
Among strategies for increasing HDL functionality, apolipoprotein A-I (apoA-I)-based infusion therapies have been the most promising. Reconstituted high-density lipoproteins (rHDL) are new compounds synthesized by combining apoA-I and phospholipids (PL), which can increase cholesterol efflux [17]. Among the different prototypes that have undergone clinical investigation, CSL111 was the most promising, showing positive effects on atherosclerotic plaques [18]. However, the CSL111 human study was stopped because of potential hepatic toxicity and was replaced by CSL112, which has thus far shown an improved liver safety profile [18].
In this review, we provide a systematic overview of the clinical pharmacokinetics (PK) and pharmacodynamics (PD) of CSL112, its safety profile, current clinical development status, and future perspective. Methods of the overview are reported in the Supplementary Information.
2 Structure and Mechanism of Action
2.1 Structure
CSL112 particles consist of purified human pooled plasma apoA-I molecules reconstituted into disc-shaped units (i.e., suitable for intravenous administration) by adding phosphatidylcholine (PC) and stabilizing with sucrose, which has a high batch-to-batch consistency [19]. Each moiety of CSL112, according to chemical cross-linking studies, consists of 2 apoA-I units combined with ~ 110 PC molecules (molar ratio of 55 PC per 1 apoA-I), with a molecular size of 144 kDa. The product is lyophilized and dissolved into sterile water for injection. Of note, CSL112 dosing is based on apoA-I protein content [20]. Lower PC and residual cholate levels, as compared to its prototype CSL111, confer an overall greater particle uniformity, and avoid the elevations in serum transaminase [21].
2.2 Mechanism of Action
The main mechanism of action of CSL112 is increasing CEC, modulating plaque cholesterol content and inflammation, and providing an overall atheroprotective effect (Fig. 2). CSL112 is a reconstituted HDL that joins the endogenous HDL physiological pathway enhancing its functionality. Therefore, in some stages, it is challenging to differentiate the CSL112 mechanism of action from the physiologic function of native HDL. Once infused intravenously over 2 h, CSL112 particles undergo a process of fusion-and-fission with endogenous HDL yielding enlarged HDL and a great amount of lipid-poor apoA-I HDL (i.e., pre-β1-HDL) [22]. Pre-β1-HDL acquire cholesterol from lipid-rich macrophages in the arterial wall via an apoA-I–ATP binding cassette subfamily A member 1 (ABCA1) receptor interaction, becoming charged of unesterified cholesterol (HDL-UC). Lecithin–cholesterol acyltransferase (LCAT) subsequently esterifies cholesterol into cholesteryl esters (HDL EC), which are accumulated into the HDL core, forming a mature HDL that will continue to uptake cholesterol, preferentially via different pathways such as ATP binding cassette subfamily G member 1 (ABCG1), Scavenger receptor class B type I (SR-BI), and passive diffusion. From here, cholesterol may be delivered to and excreted by the liver or intestine or passed to other lipoproteins (Fig. 1).
CSL112 plaque stabilization mechanism. *In humans, plaque volume assessments with intravascular ultrasound were evaluated with CSL111. Overall, CSL111/CSL112 has been associated with improvement in markers related to atherosclerotic plaque stabilization characteristics such as inflammatory parameters, reduces plaque lipid content and necrotic core, and increases the collagen content of the plaque fibrous cap, without a significant change in plaque volume
2.2.1 Plaque Stabilization
Reducing cholesterol content in atherosclerotic plaque promotes plaque stabilization and reduces cholesterol deposit-mediated macrophage inflammation [16]. Furthermore, cholesterol depletion from plasma membranes and lipid rafts can reduce the activation of monocytes, neutrophils, and other inflammatory cell responses [23]. Studies in apolipoprotein E-deficient mice (i.e., atherosclerosis-prone with hypercholesterolemia) treated with CSL111 showed a significant reduction in lipid content, an increase in plaque collagen levels, and a reduction in macrophage number and its inflammatory phenotype [24].
In patients with non-ST-segment elevation MI (NSTEMI), although CSL111 did not significantly reduce atheroma volume compared to placebo, assessed by means of intravascular ultrasound, significant improvement in the plaque characterization index and coronary score was observed on quantitative coronary angiography, suggesting plaque stabilization (Fig. 2) [18]. Moreover, in patients with carotid artery disease that underwent carotid endarterectomy, CSL111 did not reduce vascular cell adhesion protein-1 expression in smooth muscle cells and endothelial cells within endarterectomy sections but modulated inflammatory and fibrosis biomarkers [25]. In patients with peripheral artery disease (PAD) who underwent superficial femoral artery revascularization and atherectomy (i.e., plaque excision), compared to placebo, a single dose of CSL111 was associated with acute changes in plaque characteristics with a reduction in lipid content, macrophage size, and measures of inflammation [26]. To date, CSL112 effects on atherosclerotic plaque burden have not been evaluated in imaging or histologic studies in humans.
2.2.2 Anti-inflammatory Effects
CSL112 has shown anti-inflammatory and cardioprotective effects in post-MI-animal models and ex vivo studies, including markers of plaque instability as matrix metalloproteinase 9 and monocyte chemotactic factor 1 and proinflammatory cytokines interleukin-1β [25, 27, 28]. Furthermore, in ex vivo models, CSL112 has been associated with the inactivation of lipid hydroperoxides in oxidized LDL by means of two small HDL species generated by the interaction of CSL112 and native HDL [22].
3 Pharmacokinetics, Pharmacodynamics, and Safety
3.1 Phase 1 Studies
CSL112 has been studied in two Phase 1 trials, a single-dose study (SAD, NCT01129661) and a multiple-ascending-dose trial (MAD, NCT01281774) (Table 1) [29, 30]. Because CSL112 is a reconstituted drug that potentiates a physiologic mechanism such as CEC, it can be challenging to differentiate the PK and PD drug effects from the native HDL levels and function. Therefore, to evaluate the net effect of CSL112, PK and PD parameters were adjusted for baseline levels (i.e., before CSL112 administration). The PK parameters evaluated were apoA-I (via immuno-nephelometric method run on a Roche Modular P analyzer [31]) and PC (via choline oxidase- DAOS [N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline] method using the Wako Phospholipids C assay [32]), the two main components of the drug, using previously validated assays. There was no indication of assay interference with CSL112 [29]. On the other hand, the PD effect was assessed by quantifying ex vivo cholesterol efflux (i.e., total CEC) and plasma lipids level variations.
The SAD study was an adaptive, double-blinded trial that randomized 58 volunteers (3:1 ratio) to CSL112 (5, 15, 40, 70, 105, 135 mg/kg) or placebo [30]. The MAD study was an adaptive, unblinded trial that randomized 36 volunteers (3:1 ratio) to CSL112 (3.4 g/once a week, 6.8 g/once a week, and 3.4 g/twice a week) or placebo. The dosing strategies and regimens employed were derived from PK modeling of the previous SAD study. The minor influence of body weight on the clearance of apoA-I and PC led to the decision to test fixed doses. Furthermore, simulations from the SAD study and evidence from other clinical studies containing apoA-I products supported the once/twice weekly administration, as it would result in minimal accumulation of apoA-I and PC [33, 34]. The goal was to reach an exposure above 500 mg/h/dL for the baseline‐corrected apoA‐I AUC0–last and above ~ 30 mg/dL for the baseline‐corrected apoA‐I Cmax, which appeared to be sufficient to obtain a ~ 10% increase in CEC [30]. Several clinical studies have shown that a higher CEC is associated with lower adverse cardiovascular outcomes. Every standard deviation increase of CEC is potentially associated with a 20% risk reduction [35].
3.1.1 Pharmacokinetics
After the CSL112 infusion, there was a rapid increase in apoA-I and PC. Apolipoprotein A-I (ApoA-I) maximal concentration (Cmax) increased proportionally to the CSL112 dose in the SAD (Cmax [mean ± SD] from 0.17 ± 0.06 g/L for the 5 mg/kg dose and to 2.81 ± 0.45 g/L in the 135 mg/kg dose) and MAD (Cmax [mean ± SD] from 0.66 ± 0.07 g/L in the first dose of the 3.4 g/1week group to 1.63 ± 0.38 g/L in the first dose of 6.8 g weekly group) studies. Furthermore, drug exposure was dose proportional (AUC0–last [mean ± SD] 197 ± 192 mg h/dL in the 5 mg/kg group to 11993 ± 4055 mg h/dL in the 135 mg/kg group) [30].
In both studies, regardless of the dose, peak plasma concentration was reached at the end of the infusion (time to Cmax [Tmax] ~ 2 h), followed by a diphasic decline. Moreover, apoA-I levels remained 3-fold above the baseline for ≥ 3 days and ≥ 5 days per dose > 70 mg/kg. The MAD study showed no evidence of drug accumulation in the once/week regimen (3.4 or 6.8 g/1-week). However, the bi-weekly administration (3.4 g/2-week) was associated with some degree of apoA-I accumulation. Similar accumulation patterns were found with PC. The apoA-I and PC ratio of AUC (RAUC) for the 3.4g/2-week group, between the last and the first dose, was ~ 2 [29].
In the MAD study, mean apoA-I half-life (t½) was highly variable in the 3.4 g dose groups (19.3 h after the first infusion of 3.4 g/2-week to 92.8 h at the last infusion of the 3.4 g/1-week group). In contrast, less variability was found in the 6.8 g/1-week group (from 39.7 to 60.8 h). These differences in t½ findings could be the consequence of endogenous apoA-I level fluctuations, sex imbalance between groups, and the small sample size of the studies. A previous radiolabeled HDL study reported an apoA-I t½ between 64.8 to 84 h [36].
Phosphatidylcholine half-life had broad variability (after the administration of all doses), again, possibly as a consequence of the limited collection of these data. Ultimately, the mean steady-state volume of distribution for apoA-I varied from 5.6 to 9.7 L, suggesting the movement of a small quantity of drug out of the plasma.
3.1.2 Pharmacodynamics
CSL112 infusion caused an increase of total CEC by ≤ 192 ± 40%. The relationship between drug administration and effect is supported by the correlation between the CEC area under the effect time curve (AUEC) and apoA-I AUC. Cholesterol efflux capacity peaked at 2 h from the infusion and returned to baseline by 72 h. The ABCA1-dependent efflux capacity increased ≤ 630 ± 421%. No saturation of AUEC for cholesterol efflux was found with the apoA-I exposure tested [30].
Pre-β1-HDL (i.e., very small HDL [HDL-VS]) increased by ≤ 3596 ± 941%, with peaks at 2 h, except for 135 mg/kg dose, which occurred at 8 h. Very small HDL levels increased accordingly to ascending CSL112 doses, with a linear relationship between apoA-I AUC0–24 and HDL-VS AUEC0–24. In particular, each group noted some degree of HDL-VS accumulation in the MAD study. Overall, there was a good correlation between HDL-VS levels and ABCA1-mediated efflux (r2 = 0.54), which was expected as apoA-I stimulates this pathway of cholesterol reabsorption.
Total cholesterol (TC) in plasma increased in a dose-dependent manner, presenting a clear time lag with CSL112 administration, which peaked between 4 and 24 h following the infusion. Total cholesterol elevation was driven by a rapid and sustained elevation in HDL-C (up to 81 ± 16.5%, with a peak of HDL-C within 24–48 h remaining elevated up to more than 72 h) with no change in non-HDL cholesterol. There was a consistent linear relationship between HDL-C AUEC0–72 and apoA-I exposure (AUC0–72). High-density lipoprotein cholesterol concentration progressively increased before each infusion, suggesting an accumulation during the study.
The rapid increase in HDL-C, seen at 2 h, was mainly due to unesterified cholesterol (HDL-UC) accumulating into HDL particles. High-density lipoprotein cholesterol peaked around 2–4 h from the beginning of the infusion and remained elevated for 48 or more hours. In contrast, HDL-esterified cholesterol (HDL-EC) peaked at 24 h and remained elevated for 72 h or more. The data suggest that cholesterol is rapidly displaced from cells to HDL particles (in the form of HDL UC) and subsequently undergoes a process of esterification (HDL EC).
Ultimately, CSL112 did not appear to increase proatherogenic lipids, as no relevant changes in lipid markers such as apolipoprotein B (apoB, primary apolipoprotein of LDL-C and other particles) and triglycerides occurred.
3.1.3 Safety
In Phase 1 studies, the most frequently reported AEs were vessel puncture site hematoma (n = 18), headache (n = 13), and infusion-site hematoma (n = 11). Overall, there were no deaths or serious adverse events (SAEs) during the SAD and MAD studies, and no AEs were directly related to treatment with CSL112 [36]. In contrast to CSL111, there was no evidence of hepatic toxicity assessed by liver enzymes or bilirubin levels. Furthermore, there were no significant changes in clinical or laboratory data in subjects to whom CSL112 was administered. In particular, no evidence of viral transmission or apoA-I–specific autoantibodies was found.
3.1.4 Special Populations
3.1.4.1 Volunteer with Moderate Renal Impairment
Although most apoA-I catabolism occurs in the liver, a small amount undergoes renal clearance [37,38,39]. However, previous Phase 1 trials included only volunteers with normal renal function. Therefore, a Phase 1 double-blind, placebo-controlled SAD study was conducted to assess CSL112 safety and PK/PD profile in volunteers with moderate chronic kidney disease (CKD, estimated glomerular filtration rate [eGFR] 30–60 mL/min/1.73 m2) compared to those without CKD [38, 39]. Patients were classified according to baseline renal function (16 with moderate CKD and 16 without CKD) into 4 cohorts and randomized (3:1 ratio) to 2 or 6 g of CSL112 or placebo [39].
ApolipoproteinA-I and PC plasma concentration-time profiles were similar between groups, with a dose-dependent increase in Cmax for apoA-I and independently from the baseline renal functional status of the patients, suggesting no difference in CSL112 exposure according to baseline renal function. Plasma clearance of apoA-I seems unaffected by lower eGFR, with an excreted fraction in urine during the first 48 h < 1% across all doses and renal function.
Sucrose, a sugar compound, is a main component of CSL112 and is excreted by the kidney. After the CSL112 infusion, a dose-dependent increase of sucrose was noted in both plasma and urine. Concentration-time profiles indicated that volunteers with moderate CKD have a slower elimination when compared to those without CKD. However, the 2 and 6 g groups excreted sucrose almost entirely within 24–48 h, respectively.
After the infusion of CSL112 at 6 g, regardless of the baseline renal function, there was an intense dose-dependent 4-fold elevation of ABCA1-dependent, a 2-fold elevation of ABCA1-independent CEC, and an increase in pre-β-HDL levels. Moreover, both groups presented similar AUEC0–24 in terms of cholesterol efflux. Total cholesterol and HDL-C increased in a dose-dependent way in both groups, with 2 h peak of HDL UC followed by 24 h peak of HDL EC. CSL112 was not associated with changes in other lipid components or inflammation parameters [38].
CSL112 exhibits a similar safety and tolerability profile in patients with or without CKD. Nevertheless, 2 of 6 subjects with moderate CKD who received the 6 g dose presented a slight (1.5× upper limit of normal [ULN]) and transient (< 24 h) increase in total bilirubin.
3.1.4.2 Japanese Volunteers
The early CSL112 Phase 1 trials were performed mostly in White males (97.2%), with a different incidence of CAD, post-MI AEs, and lipoprotein metabolisms compared to other ethnic groups. Therefore, a CSL112 Phase 1 study was conducted on 34 Japanese volunteers to evaluate the PK/PD profiles and safety of different CSL112 doses (2, 4, and 6 g single dose) compared to placebo in oriental races (Table 1) [40]. Furthermore, they randomized White subjects to either 6 g or placebo (one administration only) to compare the two populations (6 g in Japanese vs 6 g in White).
Apolipoprotein A-I levels in the Japanese population increased in a non-linear and dose-dependent way, peaking at 2 h and decreasing in a biphasic fashion. Of note, they remained above the baseline for 72 h (in the 2 and 4 g groups) and 144 h (in the 6 g group). Apolipoprotein A-I mean baseline-corrected AUC0–72 went from 840 mg h/dL in the 2 g cohort to 6490 mg/h/dL in the 6 g cohort, highlighting a dose-dependent exposure of CSL112. Overall, Japanese volunteers experience a similar PK profile compared to White subjects. The geometric mean ratios (Japanese:White) for plasma apoA-I AUC0–72 and Cmax were 1.08 and 0.945, respectively.
The total cholesterol efflux increase and the ABCA1-dependent cholesterol efflux increase in Japanese and White subjects were similar (total CEC: 2.74-fold vs 2.92-fold and ABCA1-dependent CEC: 7.29-fold vs 8.36-fold, respectively), together with pre-β1-HDL, and HDL-C levels. No main alterations in non-HDL-C, apoB, LDL-C, or triglycerides occurred in any group. Ultimately, there was a similar safety profile between Japanese and Whites, except for the presentation of 3 hypersensitivity cases (i.e., a non-serious rash) that resolved without treatment, but only one was assessed as being related to the study treatment. Two cases of non-clinically relevant electrocardiogram changes were observed.
3.2 Phase 2 Studies
Three Phase 2 studies, including a Phase 2b study, have been conducted with CSL112 (Table 2) to assess the safety and PK/PD profile in patients with stable CAD or post-MI.
3.2.1 Patients with Stable CAD
Tricoci et al, conducted a single ascending, double-blinded, placebo-controlled trial in patients with stable CAD [21]. A total of 45 patients were randomized first into three dose groups and second to either CSL112 (1.7 g [n = 7], 3.4 g [n = 12], and 6.8 g [n = 14]) or placebo (n = 11) (3:1 ratio). Randomization was stratified for normal (eGFR 90 mL/min/1.73 m2) or mild (eGFR between 60 and 90 mL/min/1.73 m2). All patients were on dual antiplatelet therapy (DAPT, i.e., aspirin plus clopidogrel or prasugrel), but patients on anticoagulant therapy were excluded. There were no serious study-drug-related adverse but mild adverse events, including infusion-site-related AE and vessel puncture-site hematoma. There were no differences in the rate of AEs between patients with normal compared with mild renal dysfunction. Furthermore, no impairment of hepatic function was observed. Still, a slight increase of serum creatinine was seen in all groups (including placebo), probably because blood sampling was performed during fasting. No seroconversion to any virus or production of anti-CSL112/apoA-I autoantibodies was reported.
Pharmacokinetic and PD profiles were similar to Phase 1 trials. Apolipoprotein A-I levels reached the maximal concentration at 2 h, and Cmax and AUC increased in a dose dependent manner (Table 2). CSL112 induced a fast dose-dependent increase in CEC (up to 3.1-fold higher than placebo).
Total cholesterol and HDL-C increased dose dependently after the infusion of CSL112, with a peak observed at 8 h, without further changes in other lipids biomarkers.
3.2.2 AEGIS-I Trial
The ApoA-I Event Reducing in Ischemic Syndromes I (AEGIS-I) trial was a Phase 2b multicenter, double-blind, placebo-controlled, dose-ranging randomized trial, conducted in 1258 patients with a recent MI (within 7 days from randomization), with either normal (n = 578) or mildly (n = 680) impaired renal function (Table 2) [41]. Co-primary endpoints were renal or hepatic toxicity at 29 days’ follow-up. Renal toxicity was defined as an increase ≥ 1.5× in serum creatinine from baseline or new-onset requirement for renal replacement therapy, with a non-inferiority margin of ≤ 5%. Hepatic toxicity was defined as a >3 ULN increase of alanine transaminase (ALT) or total bilirubin, with a non-inferiority margin of ≤ 4%. Key secondary endpoints included bleeding and MACE (composite of CV death, nonfatal MI, ischemic stroke, or hospitalization for unstable angina) at 1 year. After stratification by baseline renal function, patients were randomized (1:1:1 ratio) to either low-dose CSL112 (2 g of apoA-I/week), high-dose CSL112 (6 g of apoA-I/week), or placebo for 4 weeks. Between 92 and 95% of the patients were on DAPT and 8%–10% on anticoagulants. At a median follow-up of 7.5 months, CSL112 was not inferior compared to placebo in terms of hepatic (CSL112 2 g vs placebo: 1 vs 0%, 95% CI [− 0.1 to 2.5]; pnoninferiority = 0.12 and CSL112 6 g vs placebo: 0.5 vs 0%, 95% CI [− 0.5 to 1.7]; pnoninferiority = 0.50) or renal toxicity (CSL112 2 g: 0 vs 0.2%, 95% CI [− 1.4 to 0.7]; pnoninferiority = 0.50 and CSL112 6 g: 0.7 vs 0.2%, 95% CI [− 0.7 to 1.9]; pnoninferiority = 0.62). At 12 months, there were no significant differences in MACE between CSL112 and placebo groups, CSL112 2 g and placebo (6.4 vs 5.5%, HR 1.18; 95% CI [0.67–2.05]; p = 0.57) or high dose CSL112 6 g and placebo (5.7 vs 5.5%, HR 1.02; 95% CI [0.57–1.80]; p = 0.97). There were no significant differences in MACE components between CSL112 2 or 6 g and placebo, except for cardiovascular death (CSL112 6 g: 1% vs placebo: 0%; p = 0.0477). Moreover, there were no differences in bleeding events, SAEs, drug hypersensitivity, and infusion site reactions. A dose-dependent increase in apoA-I concentration, total CEC, and ABCA1-dependent CEC (2.51-fold in 2 g dose and 1.78-fold in 6 g dose) were observed (Table 2).
ApoA-I Event Reducing in Ischemic Syndromes I trial represents the largest trial conducted so far assessing the safety of CSL112 in patients with an MI in the past 7 days. Overall, safety outcomes were reassuring, suggesting no renal and hepatic toxicity without signals of other SAEs. Both doses of CSL112 were associated with a significant increase in CEC. As a Phase 2b trial, the analyses of clinical events were underpowered and not adjusted for multiple comparisons. Therefore, clinical outcomes should be evaluated cautiously and considered hypothesis generating. In particular, in the difference in cardiovascular death between CSL112 6 g dose and placebo, there was a low rate of events with no clustering of deaths in proximity to the CSL112 infusion, and indeterminant causes of death were inputted as cardiovascular death.
Preliminary data from ongoing AEGIS-I ex vivo sub-studies have suggested that CSL112 infusion promotes hepatocytes cholesterol uptake and apoA-I exchange rate (i.e., biomarker of HDL functionality) compared to placebo [42, 43].
3.2.3 AEGIS-I Trial PK/PD Sub-Study
A small cohort of patients (n = 63) of the AEGIS-I trial underwent PK/PD profile analysis and several biomarker assessments. Overall, the results confirmed the previous PK/PD findings (Table 2). The ApoA-I exposure was 3- to 4-fold higher in the 6 g group versus the 2 g group after the first dose and 3-fold higher after the last doses. The fact that there were slightly higher levels after the last doses compared to the first doses suggests a slight degree of accumulation. Phosphatidylcholine showed a similar PK profile but a shorter half-life, with an exposure 3-fold higher in the 6 g group than in the 2 g group. There were no substantial differences in apoA-I and PC PK profiles among patients with normal, mild, or moderately reduced renal function (Fig. 3) [44]. The exposure-response relationship after CSL112 infusion was analyzed in the AEGIS I PK/PD sub-study [44]. A 2-fold increase in total CEC and a 3-fold increase in ABCA1-dependent CEC with respect to baseline was observed at 2 h in the 2 g dose group. At the same time, a 3-fold increase in total CEC and a 6-fold increase in ABCA1-dependent CEC with respect to baseline occurred in the 6 g dose group.
Changes in apolipoprotein A-I and phosphatidylcholine following CSL112 infusion. Presented are mean ± standard deviation of baseline-corrected plasma concentration-time profiles for apolipoprotein A-I (apoA-I) (A) and phosphatidylcholine (PC) (B). Total study population: 63 (placebo [n = 18], 2 g CSL112 [n = 24], 6 g CSL112 [n = 21]). Normal renal function: 36 patients; Mild renal impairment: 26; Moderate renal impairment: Reproduced with permission from Gibson et al. [44]
The 2-h time point (end of infusion) represents the Tmax R (i.e., time to reach maximal response) for ABCA1-dependent and ABCA1-independent CEC in the 2 g and the 6 g CSL112 doses. In the 6 g group, ABCA1-dependent and ABCA1-independent CEC returned to baseline after 48 h. However, in the 2 g group, ABCA1-dependent CEC returned to baseline after 12 h and ABCA1-independent CEC after 24 h, suggesting an exposure-response relationship between CEC and CSL112 dose.
There was a strong correlation between CSL112 dose and ABCA1-independent CEC AUEC0–24 (R2=0.800) and total CEC AUEC0–24 (R2=0.757). Notably, the Rmax (maximal response) of ABCA1-independent CEC was dose proportional, while it appeared to be saturated for ABCA1-dependent and total CEC. The cause of the saturation was deemed to be assay saturation, noted in samples collected in 2 and 6 g groups at 2 h. Ultimately, there was a positive linear relationship between the increasing CSL112 dose-dependent apoA-I exposure and the total, ABCA1-dependent, and ABCA1-independent CEC response, consistent after the first and last infusion (Fig. 4) [44].
Correlation between apolipoprotein A-I and CEC exposure following infusion of CSL112. Presented are the linear relationships between pre-dose-corrected apolipoprotein. A-I (apoA-I) exposure, area under the curve in the first 24 h post-infusion (AUC0–24), and pre-dose-corrected cholesterol efflux capacity (CEC), area under the effect curve in the first 24 h (AUEC0–24): total (A), ABCA1-dependent (B), and ABCA1-independent CEC (C), following the first and last infusions of CSL112. Reproduced with permission from Gibson et al. [44]
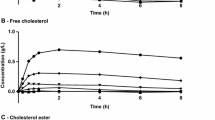
High-density lipoprotein cholesterol peaked between 2 to 6 h, returning to baseline after 48 h in the 2 g group and 96 h in the 6 g group (Fig. 5). There was a transient 24-h increase in total cholesterol without an increase in atherogenic lipids or lipoproteins. Among the inflammatory, metabolic, and cardiac biomarkers evaluated, there were no significant differences between CSL112 and placebo. The authors claimed that the observed variability in the evaluated biomarkers could potentially be related to the post-MI condition rather than to a CSL112 effect.
Elevations in cholesterol efflux capacity following CSL112 infusion. Presented are mean ± standard deviation baseline- (first infusion) and pre-dose-corrected (last infusion) total (A), ABCA1-dependent (B), and ABCA1-independent (C) cholesterol efflux capacity. Total study population: 63 (placebo [n = 18], 2 g CSL112 group [n = 24], 6 g CSL112 [n = 21]). Normal renal function: 36 patients; mild renal impairment: 26 patients; moderate renal impairment: 1 patient. Reproduced with permission from Gibson et al. [44]
3.2.4 CSL112-2001 Trial
The CSL112-2001 trial (A Study of CSL112 in Adults With Moderate Renal Impairment and Acute Myocardial Infarction) was a double-blinded, placebo-controlled, parallel group randomized trial that included 83 post-MI patients (within 7 days of the index event) with moderate CKD (eGFR between 45 and 60 mL/min/1.73 m2) and diabetes (Table 2) [45]. Patients were randomized to CSL112 6 g or placebo (2:1 ratio), stratified according to the CKD stage (i.e., 3a and 3b stage). The co-primary safety endpoint was renal SAE (composite of acute renal failure or renal, cortical, or papillary necrosis) and treatment-emergent acute kidney injury (AKI, sustained increase of creatinine ≥ 0.3 mg/dL from baseline). Key secondary endpoints included changes in hepatic status (> 3 ULN increase of ALT or total bilirubin). There were no differences between CSL112 6 g and placebo in AKI events (4.0 vs 14.3%, 95% CI [−0.277 to −0.025]; p = 0.180), but there was a lower rate of renal SAEs in the CSL112 group compared to placebo (1.9 vs 14.3%, 95% CI [− 0.296 to − 0.005]; p = 0.048). There were no differences between groups in hepatic status and bleeding events. In this cohort of patients with moderate CKD, CSL112 6 g was associated with similar apoA-I and CEC to the one observed in post-MI patients with normal renal function or mild CKD.
3.3 Pooled Data Analysis
Data from the Phase 1 and 2 trials have been combined in 3 different pooled data analyses to increase the study's sample size and assess the PK/PD profiles among different sub-groups [20, 40, 46].
In 2018, Gille et al. [20] performed a pooled analysis of the results from previous Phase 1 and 2 trials, including healthy volunteers and patients with stable CAD, to compare PK/PD profiles between them. A total of 137 patients were included (Phase 1 SAD, n = 57; Phase 1 MAD, n = 36; and Phase 2a, n = 44). Patients with stable CAD exhibited an impaired HDL function compared to healthy individuals, characterized by a significantly lower ABCA1-mediated CEC at baseline (p < 0.0001) despite slightly higher apoA-I levels. However, CSL112 infusion overcame the baseline HDL dysfunction in patients with CAD, and no differences were observed in apoA-I PK or pre-β1-HDL (p = 0.500) or CEC (p = 0.100). These findings suggest that the disease processes that reduce the efflux activity of endogenous apoA-I have a negligible effect on infused CSL112. When CEC was analyzed based on tertiles of apoA-I–normalized CEC (i.e., level of HDL function), there were no differences in the elevation in CEC (p = 0.100).
In 2020, Zheng et al. [46] performed a pharmacometrics analysis pooling data from previous Phase 1 (n = 4) and 2 (n = 3) clinical trials (including White and Japanese healthy volunteers, stable CAD, post-MI, and post-MI patients with moderate CKD) to characterize the population PK and exposure-response models evaluated as total and ABCA1-dependent CEC, and to assess the influence of covariates in this relationships. Overall, the models did not identify any covariate that had a clinically relevant effect. In particular, neither the body weight, sex, nor the ethnic group interfered with the desired CEC elevation associated with a 6 g dose of CSL112.
In the previously mentioned Phase 1 study performed by Zheng et al. [40], the authors performed a cross-study comparison to compare PK parameters of apoA-I across five completed CSL112 clinical trials, including healthy volunteers, stable CAD, and post-MI patients [20, 21, 29, 30, 41]. Overall, CSL112 exposure and CEC enhancement were similar in Japanese and non-Japanese subjects, which further confirmed the consistent PK/PD profiles of CSL112. The infusion of 6 g CSL112 resulted in similar median elevations of apoA-I in Japanese and White subjects and post-MI patients (2.08-, 2.33-, and 2.08-fold increase), total CEC (2.74-, 2.92- and 2.55-fold increase), and ABCA1-dependent CEC (7.29-, 8.36-, and 3.84-fold increase).
3.4 Further Investigations
3.4.1 Effects on Platelet Inhibition
In a previous study with CSL111 (predecessor compound), infusion in patients with type 2 diabetes without concomitant antiplatelet therapy, was associated with a significant reduction in the ex vivo platelet aggregation in response to multiple agonists. This effect persisted in washed platelets [47]. Moreover, in vitro studies in platelets from healthy individuals revealed that the inhibitory effects of CSL111 on platelet function were time- and dose-dependent and resulted in a widespread attenuation of platelet function and a significant reduction in thrombus formation under flow. The proposed mechanism of action was reducing cholesterol content in platelet membranes.
In a dedicated study with CSL112, platelet reactivity and bleeding risk were assessed in patients (n = 44) with stable CAD receiving dual antiplatelet therapy (i.e., aspirin plus clopidogrel [n = 37] or prasugrel [n = 7]) in a single ascending dose placebo-controlled randomized trial, comparing CSL112 infusion (1.7, 3.4, or 6.8 g dose) versus placebo [48]. Platelet aggregation was assessed by means of light transmission aggregometry using arachidonic acid, adenosine diphosphate, and collagen as agonists, at baseline and up to 48 h post-dosing. Compared to placebo, CSL112 had no meaningful time- or dose-dependent effects on maximum platelet aggregation in response to any agonist, regardless of the dose or renal function subgroup. There were no significant changes in coagulation parameters, and no differences in bleeding events were observed.
3.4.2 Interaction with Lipid-Lowering Therapies
Lipid-lowering therapies (i.e., statins and proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor) are a cornerstone in preventing recurrent events in post-MI patients. In animal models, high doses of rHDL are associated with a transient increase of hepatic enzymes. In an animal model, Beyerle et al. [49] performed a toxicity evaluation of the combination of different CSL112 doses with alirocumab (a PCSK9 inhibitor) and/or atorvastatin (a statin). Although high-dose CSL112 was associated with a non-significant elevation of liver enzymes, there was limited evidence of hepatic toxicity with the co-administration of CSL112 with alirocumab and/or atorvastatin assessed by means of liver enzymes and histology. Co-administration of the study drugs had minimal effect on their respective exposure levels and total cholesterol and HDL-C levels. To date, no dedicated investigations in humans on drug-drug interaction are available.
4 Future Directions
CSL112 has been tested in seven different Phase 1 and Phase 2 trials (Tables 1 and 2), including 1060 participants who received at least one drug dose. In contrast to its prototype, CSL112 has shown favorable safety and tolerability profile combined with robust PK/PD data that led to its study in a large-scale Phase 3 trial.
4.1 AEGIS II
The ApoA-I Event Reducing in Ischemic Syndromes II (AEGIS-II) trial is an ongoing multicenter, double-blinded, placebo-controlled, parallel group randomized controlled trial, which targets > 18,000 post-MI patients [50]. AEGIS-II will be the first trial to formally determine whether enhancing CEC by means of CSL112 infusion can reduce the rate of recurrent MACE. The studied population will include patients with spontaneous MI, evidence of multivessel stable coronary artery disease, and the presence of diabetes requiring pharmacotherapy, or ≥ 2 of the following: aged ≥ 65 years, prior MI, or PAD. Key exclusion criteria include ongoing hemodynamic instability, hepatobiliary disease, severe CKD (including dialysis), scheduled bypass surgery, and body weight below 50 kg. Patients are randomized (1:1 ratio) to receive weekly infusions of CSL112 6 g or placebo for 4 weeks, initiated prior to or on the day of discharge and within 5 days of first medical contact. All 4 infusions should be administrated 5 to 8 days apart, within 30 days. Randomization will be stratified by the type of MI (ST-segment elevation MI vs non-ST-segment elevation MI), management (invasive vs medical), and region. The primary efficacy outcome is the time to first occurrence of MACE (composite of cardiovascular death, MI, and stroke) at 90 days of follow-up. Key secondary outcomes included the total number of hospitalizations for coronary, cerebral, or peripheral ischemia at 90 days follow-up and time to first occurrence of MACE from randomization through 180 and 365 days. Safety outcomes include the number of participants with AEs through 90 days, treatment-related AEs, and SAEs through to the end of the study.
The final sample size (n = 18,231) was based on CEC-adjudicated MACE rates and was monitored during the enrollment phase. The sample size was calculated assuming CSL112 will have a 20% relative risk reduction compared to placebo MACE at 90 days’ follow-up. Assuming a 1-sided α of 0.025, 1004 confirmed MACE events will provide at least 90% power. Sample size calculation considers 3 planned interim analyses performed at 30, 50, and 70% of the target number of CEC-adjudicated events. The first and second interim analyses will assess futility, and the third interim analysis will assess efficacy.
The study had a protocol amendment because the blinded aggregate primary endpoint event rates were lower than anticipated, leading to two major changes. First, the risk profile of the study population was enhanced. The new protocol requires patients to have either pharmacologically treated diabetes mellitus or any 2 or more of the other established risk factors (aged ≥ 65 years, prior MI, or PAD) versus the original requirement that subjects have only 1 risk factor. Second, the definition of MI within the composite primary endpoint was expanded to include all MIs and not just type 1 MIs as originally designed. The rationale was to reduce a potentially negative impact on sensitivity and misclassification in accounting for MI in the primary endpoint.
5 Regulatory Affairs
After completing Phases 1 and 2, the safety and efficacy of CSL112 need to be proved in the ongoing Phase 3 AEGIS-II trial before being evaluated by regulatory agencies. The trial enrollment was interrupted during the COVID-19 pandemic causing a delay in the trial timelines. The study enrollment was completed on November 21st, 2022, and the estimated primary endpoint (MACE) completion is expected for March 2023. Furthermore, the end of the study and key secondary endpoints (components of MACE and safety) are scheduled for late December 2023 [51].
6 Conclusion
Prevention of recurrent adverse CV events is an unmet clinical need, representing an increasing cause of morbidity and mortality, particularly during the trimester following an MI. Among all the available targets, HDL represents an interesting therapeutic approach because of its strong relationship with CAD. After the failure of several trials focused on increasing circulating HDL levels, the target has now moved towards enhancing the function of HDL by means of reconstituted HDL. Following a decade of investigations, CSL112 has proven to be a safe and well-tolerated compound showing promising results in post-MI patients in Phase 2b AEGIS-I trial. CSL112 is being studied in the ongoing large-scale Phase 3 AEGIS-II trial to determine its efficacy in reducing MACE in post-MI high-risk patients (Table 3).
Availability of data and material
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Change history
14 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40262-023-01277-9
References
Ortega-Paz L, Capodanno D, Angiolillo DJ. Canakinumab for secondary prevention of coronary artery disease. Future Cardiol. 2021;17(3):427–42.
Brugaletta S, Gomez-Lara J, Ortega-Paz L, Jimenez-Diaz V, Jimenez M, Jimenez-Quevedo P, et al. 10-year follow-up of patients with everolimus-eluting versus bare-metal stents after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77(9):1165–78.
Coughlan JJ, Maeng M, Räber L, Brugaletta S, Aytekin A, Okkels Jensen L, Bär S, Ortega-Paz L, Laugwitz KL, Madsen M, Heg D, Sabaté M, Kufner S, Warnakula Olesen KK, Kastrati A, Windecker S, Cassese S. Ten-year patterns of stent thrombosis after percutaneous coronary intervention with newversus early-generation drug-eluting stents: insights from the DECADE cooperation. Rev Esp Cardiol (Engl Ed). 2022 Apr 15:S1885-5857(22)00031-7. English, Spanish. https://doi.org/10.1016/j.rec.2022.02.003. Epub ahead of print. PMID: 35437213.
Angiolillo DJ, Galli M, Collet JP, Kastrati A, O’Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17(17):e1371–96.
Shotan A, Blondheim DS, Gottlieb S, Kazatsker M, Frimerman A, Shochat M, et al. Comparison of outcome of recurrent versus first ST-segment elevation myocardial infarction (from national Israel surveys 1998 to 2006). Am J Cardiol. 2011;107(12):1730–7.
Smolina K, Wright FL, Rayner M, Goldacre MJ. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5(4):532–40.
Vandenberghe D, Albrecht J. The financial burden of non-communicable diseases in the European Union: a systematic review. Eur J Public Health. 2020;30(4):833–9.
Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport. Circ Res. 2019;124(10):1505–18.
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15.
Capodanno D, Mehran R, Gibson CM, Angiolillo DJ. CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I: safety and tolerability profiles and implications for management in patients with myocardial infarction. Expert Opin Investig Drugs. 2018;27(12):997–1005.
Group HTRC, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217–27.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–93.
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35.
Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3(7):507–13.
Guerin M, Silvain J, Gall J, Darabi M, Berthet M, Frisdal E, et al. Association of serum cholesterol efflux capacity with mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2018;72(25):3259–69.
Kingwell BA, Nicholls SJ, Velkoska E, Didichenko SA, Duffy D, Korjian S, et al. Antiatherosclerotic effects of CSL112 mediated by enhanced cholesterol efflux capacity. J Am Heart Assoc. 2022;11(8): e024754.
Huang J, Wang D, Huang LH, Huang H. Roles of Reconstituted High-Density Lipoprotein Nanoparticles in Cardiovascular Disease: A New Paradigm for Drug Discovery. Int J Mol Sci. 2020 Jan 23;21(3).
Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297(15):1675–82.
Diditchenko S, Gille A, Pragst I, Stadler D, Waelchli M, Hamilton R, et al. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler Thromb Vasc Biol. 2013;33(9):2202–11.
Gille A, D’Andrea D, Tortorici MA, Hartel G, Wright SD. CSL112 (apolipoprotein A-I [human]) enhances cholesterol efflux similarly in healthy individuals and stable atherosclerotic disease patients. Arterioscler Thromb Vasc Biol. 2018;38(4):953–63.
Tricoci P, D’Andrea DM, Gurbel PA, Yao Z, Cuchel M, Winston B, et al. Infusion of reconstituted high-density lipoprotein, CSL112, in patients with atherosclerosis: safety and pharmacokinetic results from a phase 2a randomized clinical trial. J Am Heart Assoc. 2015;4(8): e002171.
Didichenko SA, Navdaev AV, Cukier AM, Gille A, Schuetz P, Spycher MO, et al. Enhanced HDL functionality in small HDL species produced upon remodeling of HDL by reconstituted HDL, CSL112: effects on cholesterol efflux, anti-inflammatory and antioxidative activity. Circ Res. 2016;119(6):751–63.
Wang SH, Yuan SG, Peng DQ, Zhao SP. High-density lipoprotein affects antigen presentation by interfering with lipid raft: a promising anti-atherogenic strategy. Clin Exp Immunol. 2010;160(2):137–42.
Murphy AJ, Funt S, Gorman D, Tall AR, Wang N. Pegylation of high-density lipoprotein decreases plasma clearance and enhances antiatherogenic activity. Circ Res. 2013;113(1):e1–9.
Nasr H, Torsney E, Poston RN, Hayes L, Gaze DC, Basser R, et al. Investigating the effect of a single infusion of reconstituted high-density lipoprotein in patients with symptomatic carotid plaques. Ann Vasc Surg. 2015;29(7):1380–91.
Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103(10):1084–91.
Richart AL, Reddy M, Khalaji M, Natoli AL, Heywood SE, Siebel AL, et al. Apo AI nanoparticles delivered post myocardial infarction moderate inflammation. Circ Res. 2020;127(11):1422–36.
Didichenko SA, Adam J, Navdaev AV, Wong M, Alhamdoosh M, Saxenhofer M, et al. Abstract 10491: CSL112 suppresses inflammation in human monocyte-derived macrophages. Circulation. 2021;144(Suppl_1):A10491.
Easton R, Gille A, D’Andrea D, Davis R, Wright SD, Shear C. A multiple ascending dose study of CSL112, an infused formulation of ApoA-I. J Clin Pharmacol. 2014;54(3):301–10.
Gille A, Easton R, D’Andrea D, Wright SD, Shear CL. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34(9):2106–14.
Roche. cobas® 8000 modular analyzer series. 2023 [cited 2023 01 January]. Available from: https://diagnostics.roche.com/us/en/products/systems/cobas-8000-analyzer-series-sys-128.html.
Wako. Phospholipids 2017 [cited 2023 January 31]. Available from: https://www.cedarlanelabs.com/Contents/Files?filePath=pdf/Wako%20Chemicals%20-%20Phospholipids%20Brochure.pdf.
Tardif JC, Heinonen T, Noble S. High-density lipoprotein/apolipoprotein A-I infusion therapy. Curr Atheroscler Rep. 2009;11(1):58–63.
Tardif JC. Emerging high-density lipoprotein infusion therapies: fulfilling the promise of epidemiology? J Clin Lipidol. 2010;4(5):399–404.
Lee JJ, Chi G, Fitzgerald C, Kazmi SHA, Kalayci A, Korjian S, et al. Cholesterol efflux capacity and its association with adverse cardiovascular events: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8: 774418.
Fidge N, Nestel P, Ishikawa T, Reardon M, Billington T. Turnover of apoproteins A-I and A-II of high density lipoprotein and the relationship to other lipoproteins in normal and hyperlipidemic individuals. Metabolism. 1980;29(7):643–53.
Georgila K, Vyrla D, Drakos E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers (Basel). 2019 Aug 1;11(8).
Gille A, Duffy D, Tortorici MA, Wright SD, Deckelbaum LI, D’Andrea DM. Moderate renal impairment does not impact the ability of CSL112 (apolipoprotein A-I [Human]) to enhance cholesterol efflux capacity. J Clin Pharmacol. 2019;59(3):427–36.
Tortorici MA, Duffy D, Evans R, Feaster J, Gille A, Mant TGK, et al. Pharmacokinetics and safety of CSL112 (Apolipoprotein A-I [Human]) in adults with moderate renal impairment and normal renal function. Clin Pharmacol Drug Dev. 2019;8(5):628–36.
Zheng B, Goto S, Clementi R, Feaster J, Duffy D, Dalitz P, et al. Effect of CSL112 (apolipoprotein A-I [human]) on cholesterol efflux capacity in Japanese subjects: findings from a phase I study and a cross-study comparison. Clin Transl Sci. 2022;15(10):2331–41.
Michael Gibson C, Korjian S, Tricoci P, Daaboul Y, Yee M, Jain P, et al. Safety and tolerability of CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I, after acute myocardial infarction: the AEGIS-I trial (ApoA-I event reducing in ischemic syndromes I). Circulation. 2016;134(24):1918–30.
Velkoska E, Greene B, Collins HL, Adelman S, Mears J, Duffy D, et al. Abstract 11305: CSL112 (apolipoprotein A-I (human)) infusion in post myocardial infarction patients promotes hepatocyte cholesterol uptake ex vivo. Circulation. 2022;146(Suppl_1):A11305.
Didichenko SA, Velkoska E, Navdaev AV, Greene BH, Lorkowski SW, Mears JJ, et al. Abstract 10220: CSL112 (apolipoprotein A-I (human)) infusion rapidly increases ApoA-I exchange rate via specific serum amyloid-poor HDL sub-populations when administered to patients post myocardial infarction. Circulation. 2021;144(Suppl_1):A10220.
Gibson CM, Kazmi SHA, Korjian S, Chi G, Phillips AT, Montazerin SM, et al. CSL112 (apolipoprotein A-I [human]) strongly enhances plasma Apoa-I and cholesterol efflux capacity in post-acute myocardial infarction patients: a PK/PD substudy of the AEGIS-I trial. J Cardiovasc Pharmacol Ther. 2022;27:10742484221121508.
Gibson CM, Kerneis M, Yee MK, Daaboul Y, Korjian S, Mehr AP, et al. The CSL112-2001 trial: safety and tolerability of multiple doses of CSL112 (apolipoprotein A-I [human]), an intravenous formulation of plasma-derived apolipoprotein A-I, among subjects with moderate renal impairment after acute myocardial infarction. Am Heart J. 2019;208:81–90.
Zheng B, Duffy D, Tricoci P, Kastrissios H, Pfister M, Wright SD, et al. Pharmacometric analyses to characterize the effect of CSL112 on apolipoprotein A-I and cholesterol efflux capacity in acute myocardial infarction patients. Br J Clin Pharmacol. 2021;87(6):2558–71.
Calkin AC, Drew BG, Ono A, Duffy SJ, Gordon MV, Schoenwaelder SM, et al. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation. 2009;120(21):2095–104.
Gurbel PA, Tantry US, D’Andrea D, Chung T, Alexander JH, Bliden KP, et al. Evaluation of potential antiplatelet effects of CSL112 (apolipoprotein A-I [human]) in patients with atherosclerosis: results from a phase 2a study. J Thromb Thrombolysis. 2018;45(4):469–76.
Beyerle A, Greene B, Dietrich B, Kingwell BA, Panjwani P, Wright SD, et al. Co-administration of CSL112 (apolipoprotein A-I [human]) with atorvastatin and alirocumab is not associated with increased hepatotoxic or toxicokinetic effects in rats. Toxicol Appl Pharmacol. 2021;1(422): 115557.
Gibson CM, Kastelein JJP, Phillips AT, Aylward PE, Yee MK, Tendera M, et al. Rationale and design of ApoA-I event reducing in ischemic syndromes II (AEGIS-II): a phase 3, multicenter, double-blind, randomized, placebo-controlled, parallel group study to investigate the efficacy and safety of CSL112 in subjects after acute myocardial infarction. Am Heart J. 2021;231:121–7.
U.S. National Institutes of Health. Study to investigate CSL112 in subjects with acute coronary syndrome—full text view [Internet]. Study to investigate CSL112 in subjects with acute coronary syndrome—full text view. ClinicalTrials.gov. 2023 [cited 2023Jan31]. Available from: https://clinicaltrials.gov/ct2/show/NCT03473223.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funds were used in the preparation of this manuscript.
Conflict of interest
Dr. Capodanno has disclosed receiving fees from Amgen, Chiesi, Daiichi Sankyo, Sanofi and Terumo. Dr. Mehran reports institutional research payments from Abbott, Abiomed, Allevi-ant Medical, AM-Pharma, Applied Therapeutics, Arena, AstraZeneca, BAIM, Bayer, Beth Israel Deaconess, Biosensors, Biotronik, Boston Scientific, Bristol-Myers Squibb, CardiaWave, CellAegis, CeloNova, CERC, Chiesi, Concept Medical, CSL Behring, Cytosorbents, DSI, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe AG, Magenta, Medtronic, Novartis, OrbusNeich, Philips, RenalPro, Vivasure, Zoll; personal fees from Cine-Med Research, WebMD; consulting fees paid to the institution from Abbott, Janssen, Medtronic, Novartis; Equity <1% in Applied Therapeutics, Elixir Medical, STEL, CONTROLRAD (spouse); Scientific Advisory Board for American Medical Association, American College of Cardiology (Board of Trustees Member), Society for Cardiovascular Angiography & Interventions (Women in Innovations Committee Member), JAMA Associate Editor; Faculty CRF (no fee). Dr. Gibson has received research grant support from Angel Medical Corporation, Bayer Corp, CSL Behring, Janssen Pharmaceuticals, Johnson & Johnson Corporation, and Portola Pharmaceuticals; and has received modest consulting monies from Amarin Pharma, Amgen, Arena Pharmaceuticals, Bayer Corporation, Boehringer Ingelheim, Boston Clinical Research Institute, Cardiovascular Research Foundation, Chiesi, CSL Behring, Eli Lilly, Gilead Sciences, Inc, Janssen Pharmaceuticals, Johnson & Johnson Corporation, The Medicines Company, Merk & Co, Inc, Novo Nordisk, Pfizer, Pharma Mar, Portola Pharmaceuticals, Sanofi, Somahlution, St Francis Hospital, Verson Corporation, and Web MD. Dr. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi and Vectura; D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, and the Scott R. MacKenzie Foundation. Dr. Luis Ortega-Paz and Dr. Salvatore Giordano have nothing to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Author contributions
Dr. LO-P, Dr. SG, and Dr. DJA: Conceptualization, Methodology, Project administration, Visualization, Writing, Reviewing and editing. Dr. DC, Dr. RM, and Dr. CMG: Reviewing and editing)
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortega-Paz, L., Giordano, S., Capodanno, D. et al. Clinical Pharmacokinetics and Pharmacodynamics of CSL112. Clin Pharmacokinet 62, 541–558 (2023). https://doi.org/10.1007/s40262-023-01224-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01224-8