Abstract
Background and Objective
We aimed to develop a meropenem population pharmacokinetic model in critically ill children receiving continuous renal replacement therapy and simulate dosing regimens to optimize patient exposure.
Methods
Meropenem plasma concentration was quantified by high-performance liquid chromatography. Meropenem pharmacokinetics was investigated using a non-linear mixed-effect modeling approach. Monte Carlo simulations were performed to compute the optimal scheme of administration, according to the target of a 100% inter-dose interval time in which concentration is one to four times above the minimum inhibitory concentration (100% fT>1–4×MIC).
Results
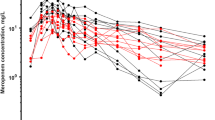
A total of 27 patients with a median age of 4 [interquartile range 0–11] years, a median body weight of 16 [range 7–35] kg receiving continuous renal replacement therapy were included. Concentration–time courses were best described by a one-compartment model with first-order elimination. Body weight (BW) produced significant effects on volume of distribution (V) and BW and continuous renal replacement therapy effluent flow rate (Qeff) produced significant effects on clearance (CL): \({V}_{i}={V}_{pop }{x (\frac{BWi}{70})}^{1}\) and \({CL}_{i}={CL}_{pop }x ({\frac{BWi}{70})}^{0.75} x ({\frac{Qeffi}{1200})}^{0.337}\), where Vpop and CLpop estimates were 32.5 L and 5.88 L/h, respectively, normalized to a 70-kg BW and median Qeff at 1200 mL/h. Using this final model and Monte Carlo simulations, for patients with Qeff over 1200 mL/h, meropenem continuous infusion was adequate in most cases to attain 100% fT>1–4xMIC. For bacterial infections with a low minimum inhibitory concentration (≤2 mg/L), meropenem intermitent administration was appropriate for patients weighing more than 20 kg with Qeff <500 mL/h and for patients weighing more than 10 kg with Qeff <100 mL/h.
Conclusions
Meropenem exposure in critically ill children receiving continuous renal replacement therapy needs dosing adjustments to the minimum inhibitory concentration that take into account body weight and the continuous renal replacement therapy effluent flow rate.





Similar content being viewed by others
References
Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis. 2015;15:46–54.
Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43:223–30.
Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, et al. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523–8.
Felton TW, Hope WW, Roberts JA. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis. 2014;79:441–7.
Thakkar N, Salerno S, Hornik CP, Gonzalez D. Clinical pharmacology studies in critically ill children. Pharm Res. 2017;34:7–24.
Groupe E, Van Vong L, Osman D, Vinsonneau C. Épuration extrarénale en réanimation adulte et pédiatrique.: recommandations formalisées d’experts sous l’égide de la Société de Réanimation de Langue Française (SRLF), avec la participation de la Société Française d’Anesthésie-Réanimation (Sfar), du Groupe Francophone de Réanimation et Urgences Pédiatriques (GFRUP) et de la Société Francophone de Dialyse (SFD): Société de Réanimation de Langue Française. Experts recommendations. Réanimation. 2014;23:714–37.
Xu X, Nie S, Zhang A, Mao J, Liu H-P, Xia H, et al. Acute kidney injury among hospitalized children in China. Clin J Am Soc Nephrol. 2018;13:1791–800.
Plötz FB, Hulst HE, Twisk JWR, Bökenkamp A, Markhorst DG, van Wijk JAE. Effect of acute renal failure on outcome in children with severe septic shock. Pediatr Nephrol. 2005;20:1177–81.
Barletta G-M, Bunchman TE. Acute renal failure in children and infants. Curr Opin Crit Care. 2004;10:499–504.
Udy AA, Roberts JA, Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013;39:2070–82.
Bridges BC, Askenazi DJ, Smith J, Goldstein SL. Pediatric renal replacement therapy in the intensive care unit. Blood Purif. 2012;34:138–48.
Shaw AR, Mueller BA. Antibiotic dosing in continuous renal replacement therapy. Adv Chronic Kidney Dis. 2017;24:219–27.
Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs. 2008;68:803–38.
Beltramo F, Dicarlo J, Gruber JB, Taylor T, Totapally BR. Renal replacement therapy modalities in critically ill children. Pediatr Crit Care Med. 2018;2:2.
Wong W-T, Choi G, Gomersall CD, Lipman J. To increase or decrease dosage of antimicrobials in septic patients during continuous renal replacement therapy: the eternal doubt. Curr Opin Pharmacol. 2015;24:68–78.
Böhler J, Donauer J, Keller F. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int. 1999;56:S24–8.
Beumier M, Casu G, Hites M, Seyler L, Cotton F, Vincent J-L, et al. β-lactam antibiotic concentrations during continuous renal replacement therapy. Crit Care. 2014;18:R105.
Mattoes HM, Kuti JL, Drusano GL, Nicolau DP. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin Ther. 2004;26:1187–98.
Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am. 2003;17:479–501.
Cohen J. Confronting the threat of multidrug-resistant Gram-negative bacteria in critically ill patients. J Antimicrob Chemother. 2013;68:490–1.
EUCAST. Clinical breakpoints and dosing of antibiotics. Available from: https://eucast.org/clinical_breakpoints/. [Accessed 22 Jun 2020].
Legrand T, Chhun S, Rey E, Blanchet B, Zahar J-R, Lanternier F, et al. Simultaneous determination of three carbapenem antibiotics in plasma by HPLC with ultraviolet detection. J Chromatogr B. 2008;875:551–6.
Schuster C, Sterz S, Teupser D, Brügel M, Vogeser M, Paal M. Multiplex therapeutic drug monitoring by isotope-dilution HPLC-MS/MS of antibiotics in critical illnesses. J Vis Exp. 2018;2:58148.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Imani S, Buscher H, Marriott D, Gentili S, Sandaradura I. Too much of a good thing: a retrospective study of β-lactam concentration-toxicity relationships. J Antimicrob Chemother. 2017;72:2891–7.
Béranger A, Oualha M, Urien S, Genuini M, Renolleau S, Aboura R, et al. Population pharmacokinetic model to optimize cefotaxime dosing regimen in critically ill children. Clin Pharmacokinet. 2018;57:867–75.
Onichimowski D, Będźkowska A, Ziółkowski H, Jaroszewski J, Borys M, Czuczwar M, et al. Population pharmacokinetics of standard-dose meropenem in critically ill patients on continuous renal replacement therapy: a prospective observational trial. Pharmacol Rep. 2020;72:719–29.
Ulldemolins M, Soy D, Llaurado-Serra M, Vaquer S, Castro P, Rodríguez AH, et al. Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother. 2015;59:5520–8.
Isla A, Rodríguez-Gascón A, Trocóniz IF, Bueno L, Solinís MÁ, Maynar J, et al. Population pharmacokinetics of meropenem in critically ill patients undergoing continuous renal replacement therapy. Clin Pharmacokinet. 2008;47:173–80.
Tan WW, Watt K, Boakye-Agyeman F, Cohen-Wolkowiez M, Mok YH, Yung CF, et al. Optimal dosing of meropenem in a small cohort of critically ill children receiving continuous renal replacement therapy. J Clin Pharmacol. 2020. https://doi.org/10.1002/jcph.1798.
Mouton JW, van den Anker JN. Meropenem clinical pharmacokinetics. Clin Pharmacokinet. 1995;28:275–86.
Tegeder I, Neumann F, Bremer F, Brune K, Lötsch J, Geisslinger G. Pharmacokinetics of meropenem in critically ill patients with acute renal failure undergoing continuous venovenous hemofiltration. Clin Pharmacol Ther. 1999;65:50–7.
Craig WA. The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis. 1997;24(Suppl. 2):S266–75.
Pistolesi V, Morabito S, Di Mario F, Regolisti G, Cantarelli C, Fiaccadori E. A guide to understanding antimicrobial drug dosing in critically ill patients on renal replacement therapy. Antimicrob Agents Chemother. 2019;63:e00583-e619.
Goldstein SL, Murry DJ, May S, Aleksic A, Sowinski KM, Blaney S. Meropenem pharmacokinetics in children and adolescents receiving hemodialysis. Pediatr Nephrol. 2001;16:1015–8.
Isla A, Maynar J, Sánchez-Izquierdo JÁ, Gascón AR, Arzuaga A, Corral E, et al. Meropenem and continuous renal replacement therapy: in vitro permeability of 2 continuous renal replacement therapy membranes and influence of patient renal function on the pharmacokinetics in critically ill patients. J Clin Pharmacol. 2005;45:1294–304.
Sime FB, Pandey S, Karamujic N, Parker S, Alexander E, Loutit J, et al. Ex vivo characterization of effects of renal replacement therapy modalities and settings on pharmacokinetics of meropenem and vaborbactam. Antimicrob Agents Chemother. 2018;62:e01306-e1318.
Saito J, Shoji K, Oho Y, Kato H, Matsumoto S, Aoki S, et al. Population pharmacokinetics and pharmacodynamic implementation of meropenem in critically ill pediatric patients. Antimicrob Agents Chemother. 2021;65:e01909-e1920.
Cojutti P, Maximova N, Pea F. Pharmacokinetics and pharmacodynamics of continuous-infusion meropenem in pediatric hematopoietic stem cell transplant patients. Antimicrob Agents Chemother. 2015;59:5535–41.
Ehmann L, Zoller M, Minichmayr IK, Scharf C, Huisinga W, Zander J, et al. Development of a dosing algorithm for meropenem in critically ill patients based on a population pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents. 2019;54:309–17.
Selig DJ, Akers KS, Chung KK, Pruskowski KA, Livezey JR, Por ED. Meropenem pharmacokinetics in critically ill patients with or without burn treated with or without continuous veno-venous hemofiltration. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.15138.
Acknowledgments
The authors thank the PICU team (physicians and nurses) that selected the children and collected the samples, making this work possible. They also thank the pharmacology laboratory of the Cochin Teaching Hospital, which analyzed the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ contributions
MT collected the data and drafted the manuscript. MT and MO conceived the study and critically revised the manuscript. EB identified pathogen agents and related MIC and also critically revised the manuscript. MT, SU, FF, and NB contributed to the acquisition, analysis, and interpretation of data and also critically revised the manuscript. IG, AB, JT, RB, FL, SR, JMT, and SB critically revised the manuscript.
Funding
Michael Thy received a grant from the “Société de Reanimation de Langue Française” supporting research on this topic.
Conflicts of Interest
The authors have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The Ethics Committee of Necker Hospital approved the study, which was registered at www.clinicaltrials.gov (NCT02539407).
Consent to Participate
Before any inclusion, written consent was obtained from children’s parent(s).
Consent for Publication
Not applicable.
Availability of Data and Material
All data generated or analyzed during this study are included in this study or its supplementary material files. Further inquiries can be directed to the corresponding author.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thy, M., Urien, S., Bouazza, N. et al. Meropenem Population Pharmacokinetics and Dosing Regimen Optimization in Critically Ill Children Receiving Continuous Renal Replacement Therapy. Clin Pharmacokinet 61, 1609–1621 (2022). https://doi.org/10.1007/s40262-022-01179-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01179-2