Abstract
Objectives
The aim of this study was to report the pharmacokinetics (PK) of caspofungin in plasma and peritoneal fluid and to identify optimal dosing strategies in septic patients with intra-abdominal infections.
Methods
Eleven patients with secondary peritonitis with septic shock received the standard dosing regimen of caspofungin. Total caspofungin plasma and peritoneal concentrations were subject to a population PK analysis using Pmetrics®. Monte Carlo simulations were performed considering the ratio of 24-h total drug exposure above the minimum inhibitory concentration (AUC24/MIC) in plasma and comparing simulated concentrations versus MIC in peritoneal fluid.
Results
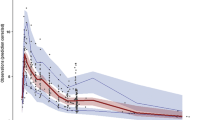
Fat-free mass (FFM) was retained in the final model of caspofungin, reporting a total clearance (standard deviation) of 0.78 (0.17) L/h and a central volume of distribution of 9.36 (2.61) L. The peritoneal fluid/plasma ratio of caspofungin was 33% on the first day of therapy (AUC24 73.92 (21.93) and 26.03 (9.88) mg*h/L for plasma and peritoneal data, respectively). Dosing simulations supported the use of standard dosing regimens for patients with an FFM < 50 kg for the most susceptible candida species (C. albicans and C. glabrata). For higher FFM, a loading dose of 70 or 100 mg, with a maintenance dose of 70 mg, reached AUC24/MIC ratios for these species.
Conclusions
There is moderate penetration of caspofungin into the peritoneal cavity (33%). For empirical treatment, a dose escalation of 100 mg loading dose on the first day is suggested for higher FFM to ensure adequate concentrations into the abdominal cavity for the most susceptible candida species.






Similar content being viewed by others
References
Pappas PG, Rotstein CMF, Betts RF, Nucci M, Talwar D, Waele JJD, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–93.
Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJC, Baron EJ, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the surgical infection society and the infectious diseases society of America. Clin Infect Dis. 2010;50:133–64.
Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31.
Bassetti M, Righi E, Ansaldi F, Merelli M, Cecilia T, De Pascale G, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014;40:839–45.
Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38:1930–45.
Paiva J-A, Pereira JM, Tabah A, Mikstacki A, de Carvalho FB, Koulenti D, et al. Characteristics and risk factors for 28-day mortality of hospital acquired fungemias in ICUs: data from the EUROBACT study. Crit Care. 2016;20:53.
Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54:1739–46.
Blot SI, Vandewoude KH, De Waele JJ. Candida peritonitis. Curr Opin Crit Care. 2007;13:195–9.
Grim SA, Berger K, Teng C, Gupta S, Layden JE, Janda WM, et al. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother. 2012;67:707–14.
Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45:737–79.
Sinnollareddy M, Peake SL, Roberts MS, Lipman J, Roberts JA. Using pharmacokinetics and pharmacodynamics to optimise dosing of antifungal agents in critically ill patients: a systematic review. Int J Antimicrob Agents. 2012;39:1–10.
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37:840–51.
Zhao Y, Prideaux B, Nagasaki Y, Lee MH, Chen P-Y, Blanc L, et al. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra-abdominal abscess model. Antimicrob Agents Chemother. 2017;61:e01009-17.
Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, et al. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients Study. Crit Care. 2015;19:33.
Jamal J-A, Roger C, Roberts JA. Understanding the impact of pathophysiological alterations during critical illness on drug pharmacokinetics. Anaesth Crit Care Pain Med. 2018;37:515–7.
Muilwijk EW, Schouten JA, van Leeuwen HJ, van Zanten ARH, de Lange DW, Colbers A, et al. Pharmacokinetics of caspofungin in ICU patients. J Antimicrob Chemother. 2014;69:3294–9.
Roger C, Wallis SC, Muller L, Saissi G, Lipman J, Brüggemann RJ, et al. Caspofungin Population Pharmacokinetics in Critically Ill Patients Undergoing Continuous Veno-Venous Haemofiltration or Haemodiafiltration. Clin Pharmacokinet. 2017;56:1057–68.
Pfaller MA, Diekema DJ, Ostrosky-Zeichner L, Rex JH, Alexander BD, Andes D, et al. Correlation of MIC with outcome for candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J Clin Microbiol. 2008;46:2620–9.
Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–703.
Bassetti M, Merelli M, Ansaldi F, de Florentiis D, Sartor A, Scarparo C, et al. Clinical and therapeutic aspects of candidemia: a five year single centre study. PLoS ONE. 2015;10:e0127534.
Montravers P, Mira J-P, Gangneux J-P, Leroy O, Lortholary O. A multicentre study of antifungal strategies and outcome of Candida spp. peritonitis in intensive-care units. Clin Microbiol Infect. 2011;17:1061–7.
Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against candida species. Antimicrob Agents Chemother. 2010;54:2497–506.
Shields RK, Nguyen MH, Press EG, Clancy CJ. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother. 2014;58:7601–5.
Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, et al. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523–8.
van der Elst KCM, Veringa A, Zijlstra Jan G, Beishuizen A, Klont R, Brummelhuis-Visser P, et al. Low caspofungin exposure in patients in the Intensive Care Unit. Antimicrob Agents Chemother. 2017;61(2):e01582-e1616.
Gioia F, Gomez-Lopez A, Alvarez ME, Gomez-García de la Pedrosa E, Martín-Davila P, Cuenca-Estrella M, et al. Pharmacokinetics of echinocandins in suspected candida peritonitis: a potential risk for resistance. Int J Infect Dis. 2020;101:24–8.
Toulouse E, Lafont B, Granier S, Mcgurk G, Bazin J-E. French legal approach to patient consent in clinical research. Anaesth Crit Care Pain Med. 2020;39:883–5.
Montravers P, Dupont H, Leone M, Constantin J-M, Mertes P-M, Laterre P-F, et al. Guidelines for management of intra-abdominal infections. Anaesth Crit Care Pain Med. 2015;34:117–30.
US FDA. Guidance for Industry: Bioanalytical Method Validation. 2018; Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM).
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63.
Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–800.
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34:467–76.
Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, et al. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn. 2013;40:189–99.
Holford NHG, Anderson BJ. Allometric size: the scientific theory and extension to normal fat mass. Eur J Pharm Sci. 2017;109:S59-64.
Colin P, Eleveld DJ, Jonckheere S, Van Bocxlaer J, De Waele J, Vermeulen A. What about confidence intervals? A word of caution when interpreting PTA simulations. J Antimicrob Chemother. 2016;71:2502–8.
Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol. 2013;51:2571–81.
Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int J Antimicrob Agents. 2017;50:352–8.
Salsé M, Gangneux J-P, Cassaing S, Delhaes L, Fekkar A, Dupont D, et al. Multicentre study to determine the Etest epidemiological cut-off values of antifungal drugs in Candida spp. and Aspergillus fumigatus species complex. Clin Microbiol Infect. 2019;25:1546–52.
Adembri C, Villa G, Rosi E, Tofani L, Fallani S, De Gaudio AR, et al. Caspofungin PK in critically ill patients after the first and fourth doses: suggestions for therapeutic drug monitoring? J Chemother. 2020;32:124–31.
Stone JA, Xu X, Winchell GA, Deutsch PJ, Pearson PG, Migoya EM, et al. Disposition of caspofungin: role of distribution in determining pharmacokinetics in plasma. Antimicrob Agents Chemother. 2004;48:815–23.
Kurland S, Furebring M, Löwdin E, Eliasson E, Nielsen EI, Sjölin J. Pharmacokinetics of caspofungin in critically ill patients in relation to liver dysfunction: differential impact of plasma albumin and bilirubin levels. Antimicrob Agents Chemother. 2019;63:11.
Bailly S, Gautier-Veyret E, Lê MP, Bouadma L, Andremont O, Neuville M, et al. Impact of loading dose of caspofungin in PK/PD target attainment for severe candidiasis infections in ICU patients—the CASPOLOAD study. Antimicrob Agents Chemother. 2020;64(12):e01545-e1620.
Grau S, Luque S, Campillo N, Samsó E, Rodríguez U, García-Bernedo CA, et al. Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis. J Antimicrob Chemother. 2015;70:2854–61.
Nguyen TH, Hoppe-Tichy T, Geiss HK, Rastall AC, Swoboda S, Schmidt J, et al. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother. 2007;60:100–6.
Märtson A-G, van der Elst KCM, Veringa A, Zijlstra JG, Beishuizen A, van der Werf TS, et al. Caspofungin weight-based dosing supported by a population pharmacokinetic model in critically ill patients. Antimicrob Agents Chemother. 2020;64:e00905-e920.
Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Alobaid AS, Hites M, Lipman J, Taccone FS, Roberts JA. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: a structured review. Int J Antimicrob Agents. 2016;47:259–68.
Betts RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, et al. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis. 2009;48:1676–84.
Cornely OA, Vehreschild JJ, Vehreschild MJGT, Würthwein G, Arenz D, Schwartz S, et al. Phase II dose escalation study of caspofungin for invasive aspergillosis. Antimicrob Agents Chemother. 2011;55:5798–803.
Würthwein G, Cornely OA, Trame MN, Vehreschild JJ, Vehreschild MJGT, Farowski F, et al. Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob Agents Chemother. 2013;57:1664–71.
Sasso M, Roger C, Sasso M, Poujol H, Barbar S, Lefrant J-Y, et al. Changes in the distribution of colonising and infecting Candida spp. isolates, antifungal drug consumption and susceptibility in a French intensive care unit: a 10-year study. Mycoses. 2017;60:770–80.
Andes D, Ambrose PG, Hammel JP, Van Wart SA, Iyer V, Reynolds DK, et al. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob Agents Chemother. 2011;55:2113–21.
Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5:161–9.
Tascini C, Sozio E, Di Paolo A, Tintori G, Leonildi A, Bertolino G, et al. Fungicidal activity and PK/PD of caspofungin as tools to guide antifungal therapy in a fluconazole-resistant C. parapsilosis candidemia. J Chemother. 2017;29:376–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by academic funding from the Nimes University Hospital (LOCAL/2016/CR-01).
Conflicts of interest/Competing interests
Jeffrey Lipman received honoraria from MSD and Pfizer and also received institution support from MSD. Claire Roger received consultancy fees from MSD, Pfizer, and Fresenius Medical Care. Nicolas Garbez, Litaty C. Mbatchi, Steven C. Wallis, Laurent Muller, Jason A. Roberts, and Jean-Yves Lefrant have no conflicts of interest to declare.
Ethics approval
Ethics approval was obtained from the local ethics committee of Nîmes (Comité de Protection des Personnes Sud-Méditerranée III 2012.02.05).
Consent to participate
Written informed consent was obtained from either the patient or their designated substitute decision maker.
Code availabitity
Pmetrics software package for R (Los Angeles, CA, USA) [32, 33].
Author contributions
CR, J-YL, and LM conceived and designed the study. CR collected data and SCW performed drug assay. NG, CR, J-YL, and JAR analysed data. NG carried out the pharmacokinetic analysis. NG, CR and LM drafted the manuscript. All authors read and approved the final manuscript.
Consent for publication
All patients provided consent for publication of their pooled, anonymized data.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garbez, N., Mbatchi, L.C., Wallis, S.C. et al. Caspofungin Population Pharmacokinetic Analysis in Plasma and Peritoneal Fluid in Septic Patients with Intra-Abdominal Infections: A Prospective Cohort Study. Clin Pharmacokinet 61, 673–686 (2022). https://doi.org/10.1007/s40262-021-01062-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01062-6