Abstract
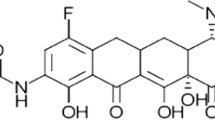
On 27 August, 2018, the US Food and Drug Administration approved eravacycline, a fluorocycline antimicrobial agent within the tetracycline class of antibacterial drugs, for the treatment of complicated intra-abdominal infections in patients aged 18 years and older. This decision was based on substantial clinical and pre-clinical data, including rigorous pharmacokinetic and pharmacodynamic work. This paper examines the in-vivo pharmacokinetic/pharmacodynamic work that led to the approval of eravacycline and explores how this important new antibiotic may be used to treat aggressive multidrug-resistant infections in the years ahead.
Similar content being viewed by others
References
Xiao XY, Hunt DK, Zhou J, Clark RB, Dunwoody N, Fyfe C, et al. Fluorocyclines. 1. 7-Fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J Med Chem. 2012;55(2):597–605.
Kang Y, Li Q, Mei L, Zhao H, Bai Y, Shen M, et al. Tetracycline resistance genes are more prevalent in wet soils than in dry soils. Ecotoxicol Environ Saf. 2018;156:337–43.
Nelson KM, Viswanathan K, Dawadi S, Duckworth BP, Boshoff HI, Barry CE, et al. Synthesis and pharmacokinetic evaluation of siderophore biosynthesis inhibitors for Mycobacterium tuberculosis. J Med Chem. 2015;58(14):5459–75.
Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, Quale J. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob Agents Chemother. 2015;59(3):1802–5.
Sutcliffe JA, O’Brien W, Fyfe C, Grossman TH. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother. 2013;57(11):5548–58.
Grossman TH, Starosta AL, Fyfe C, O’Brien W, Rothstein DM, Mikolajka A, et al. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother. 2012;56(5):2559–64.
Snydman DR, McDermott LA, Jacobus NV, Kerstein K, Grossman TH, Sutcliffe JA. Evaluation of the in vitro activity of eravacycline against a broad spectrum of recent clinical anaerobic isolates. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/aac.02206-17.
Zhao M, Lepak AJ, Marchillo K, VanHecker J, Andes DR. In vivo pharmacodynamic target assessment of eravacycline against Escherichia coli in a murine thigh infection model. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/aac.00250-17.
Thabit AK, Monogue ML, Nicolau DP. Eravacycline pharmacokinetics and challenges in defining humanized exposure in vivo. Antimicrob Agents Chemother. 2016;60(8):5072–5.
Petraitis V, Petraitiene R, Maung BBW, Khan F, Alisauskaite I, Olesky M, et al. Pharmacokinetics and comprehensive analysis of the tissue distribution of eravacycline in rabbits. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/aac.00275-18.
Connors KP, Housman ST, Pope JS, Russomanno J, Salerno E, Shore E, et al. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob Agents Chemother. 2014;58(4):2113–8.
Bassetti M, Vena A, Castaldo N, Righi E, Peghin M. New antibiotics for ventilator-associated pneumonia. Curr Opin Infect Dis. 2018;31(2):177–86.
Nation RL, Theuretzbacher U, Tsuji BT, International Society of Anti-Infective Pharmacology (ISAP). Concentration-dependent plasma protein binding: expect the unexpected. Eur J Pharm Sci. 2018;122:341–6.
Newman JV, Zhou J, Izmailyan S, Tsai L. Randomized, double-blind, placebo-controlled studies of the safety and pharmacokinetics of single and multiple ascending doses of eravacycline. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/aac.01174-18.
Thabit AK, Monogue ML, Newman JV, Nicolau DP. Assessment of in vivo efficacy of eravacycline against Enterobacteriaceae exhibiting various resistance mechanisms: a dose-ranging study and pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents. 2018;51(5):727–32.
Daoud Z, Farah J, Sokhn ES, El Kfoury K, Dahdouh E, Masri K, et al. Multidrug-resistant Enterobacteriaceae in Lebanese Hospital wastewater: implication in the one health concept. Microb Drug Resist. 2018;24(2):166–74.
Bathoorn E, Tsioutis C, da Silva Voorham JM, Scoulica EV, Ioannidou E, Zhou K, et al. Emergence of pan-resistance in KPC-2 carbapenemase-producing Klebsiella pneumoniae in Crete, Greece: a close call. J Antimicrob Chemother. 2016;71(5):1207–12.
Sheng ZK, Li JJ, Sheng GP, Sheng JF, Li LJ. Emergence of Klebsiella pneumoniae carbapenemase-producing Proteus mirabilis in Hangzhou, China. Chin Med J (Engl). 2010;123(18):2568–70.
Newman JV, Zhou J, Izmailyan S, Tsai L. Mass balance and drug interaction potential of intravenous eravacycline administered to healthy subjects. Antimicrob Agents Chemother. 2019;63:3. https://doi.org/10.1128/aac.01810-18.
Roffey SJ, Obach RS, Gedge JI, Smith DA. What is the objective of the mass balance study? A retrospective analysis of data in animal and human excretion studies employing radiolabeled drugs. Drug Metab Rev. 2007;39(1):17–43.
Koulenti D, Song A, Ellingboe A, Abdul-Aziz MH, Harris P, Gavey E, et al. Infections by multidrug-resistant Gram-negative bacteria: what’s new in our arsenal and what’s in the pipeline? Int J Antimicrob Agents. 2019;53(3):211–24.
Thaden JT, Pogue JM, Kaye KS. Role of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Virulence. 2017;8(4):403–16.
Zheng JX, Lin ZW, Sun X, Lin WH, Chen Z, Wu Y, et al. Overexpression of OqxAB and MacAB efflux pumps contributes to eravacycline resistance and heteroresistance in clinical isolates of Klebsiella pneumoniae. Emerg Microbes Infect. 2018;7(1):139.
Honore PM, Spapen HD. Eravacycline for treatment of complicated intra-abdominal infections: the fire is not ignited! Ann Transl Med. 2017;5(21):425.
Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating Gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg. 2017;152(3):224–32.
Bassetti M, Righi E. Eravacycline for the treatment of intra-abdominal infections. Expert Opin Investig Drugs. 2014;23(11):1575–84.
Solomkin JS, Gardovskis J, Lawrence K, Montravers P, Sway A, Evans D, et al. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs. meropenem in the treatment of complicated intra-abdominal infections. Clin Infect Dis. 2018. https://doi.org/10.1093/cid/ciy1029.
Tripathi PC, Gajbhiye SR, Agrawal GN. Clinical and antimicrobial profile of Acinetobacter spp.: an emerging nosocomial superbug. Adv Biomed Res. 2014;3:13.
Mohammed N, Savardekar AR, Patra DP, Narayan V, Nanda A. The 21st-century challenge to neurocritical care: the rise of the superbug Acinetobacter baumannii: a meta-analysis of the role of intrathecal or intraventricular antimicrobial therapy in reduction of mortality. Neurosurg Focus. 2017;43(5):E8.
Seifert H, Stefanik D, Sutcliffe JA, Higgins PG. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int J Antimicrob Agents. 2018;51(1):62–4.
Raz-Pasteur A, Liron Y, Amir-Ronen R, Abdelgani S, Ohanyan A, Geffen Y, et al. Trimethoprim-sulfamethoxazole vs. colistin or ampicillin-sulbactam for the treatment of carbapenem-resistant Acinetobacter baumannii: a retrospective matched cohort study. J Glob Antimicrob Resist. 2018. https://doi.org/10.1016/j.jgar.2018.12.001.
Zhang F, Bai B, Xu GJ, Lin ZW, Li GQ, Chen Z, et al. Eravacycline activity against clinical S. aureus isolates from China: in vitro activity, MLST profiles and heteroresistance. BMC Microbiol. 2018;18(1):211.
Poulakou G, Lagou S, Karageorgopoulos DE, Dimopoulos G. New treatments of multidrug-resistant Gram-negative ventilator-associated pneumonia. Ann Transl Med. 2018;6(21):423.
Mancini S, Kieffer N, Poirel L, Nordmann P. Evaluation of the RAPIDEC® CARBA NP and β-CARBA® tests for rapid detection of carbapenemase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis. 2017;88(4):293–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of Interest
Matthew W. McCarthy has no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
McCarthy, M.W. Clinical Pharmacokinetics and Pharmacodynamics of Eravacycline. Clin Pharmacokinet 58, 1149–1153 (2019). https://doi.org/10.1007/s40262-019-00767-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00767-z