Abstract
Background
Chemotherapy-induced thrombocytopenia is often a use-limiting adverse reaction to gemcitabine and cisplatin (GC) combination chemotherapy, reducing therapeutic intensity, and, in some cases, requiring platelet transfusion.
Objective
A retrospective cohort study was conducted on patients with urothelial cancer at the initiation of GC combination therapy and the objective was to develop a prediction model for the incidence of severe thrombocytopenia using machine learning.
Methods
We performed receiver operating characteristic analysis to determine the cut-off values of the associated factors. Multivariate analyses were conducted to identify risk factors associated with the occurrence of severe thrombocytopenia. The prediction model was constructed from an ensemble model and gradient-boosted decision trees to estimate the risk of an outcome using the risk factors associated with the occurrence of severe thrombocytopenia.
Results
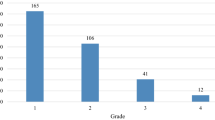
Of 186 patients included in this study, 46 (25%) experienced severe thrombocytopenia induced by GC therapy. Multivariate analyses revealed that platelet count ≤ 21.4 (×104/µL) [odds ratio 7.19, p < 0.01], hemoglobin ≤ 12.1 (g/dL) [odds ratio 2.41, p = 0.03], lymphocyte count ≤ 1.458 (×103/µL) [odds ratio 2.47, p = 0.02], and dose of gemcitabine ≥ 775.245 (mg/m2) [odds ratio 4.00, p < 0.01] were risk factors of severe thrombocytopenia. The performance of the prediction model using these associated factors was high (area under the curve 0.76, accuracy 0.82, precision 0.68, recall 0.50, and F-measure 0.58).
Conclusions
Platelet count, hemoglobin level, lymphocyte count, and gemcitabine dose contributed to the development of a novel prediction model to identify the incidence of GC-induced severe thrombocytopenia.


Similar content being viewed by others
References
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–77.
Blay JY, Le Cesne A, Mermet C, Maugard C, Ravaud A, Chevreau C, et al. A risk model for thrombocytopenia requiring platelet transfusion after cytotoxic chemotherapy. Blood. 1998;92(2):405–10.
Wang Z, Cai XJ, Chen LY, Cheng B, Shi L, Lei L, et al. Factors potentially associated with gemcitabine-based chemotherapy-induced thrombocytopenia in Chinese patients with nonsmall cell lung cancer. J Cancer Res Ther. 2018;14(Suppl):S656–60.
Takahashi N, Sunaga T, Fujimiya T, Kurihara T, Nagatani A, Yamagishi M, et al. Risk associated with severe hematological toxicity in patients with urothelial cancer receiving combination chemotherapy of gemcitabine and cisplatin. Chemotherapy. 2020;65(1–2):29–34.
Takahashi M, Takahashi K, Kaneda H, Kawaguchi T, Nagayama K. Pretreatment platelet count and neutrophil/lymphocyte ratio are predictive markers for carboplatin plus pemetrexed therapy-induced thrombocytopenia. Anticancer Res. 2021;41(11):5729–37.
Zhou S, Song B, Li C, Tang W, Zhang X, Jin X, et al. The predictive model for risk of chemotherapy-induced thrombocytopenia based on antineoplastic drugs for solid tumors in eastern China. Sci Rep. 2023;13(1):3185.
Okawa T, Mizuno T, Hanabusa S, Ikeda T, Mizokami F, Koseki T, et al. Prediction model of acute kidney injury induced by cisplatin in older adults using a machine learning algorithm. PLoS ONE. 2022;17(1): e0262021.
Bantis LE, Nakas CT, Reiser B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics. 2014;70(1):212–23.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Sony Network Communications, Inc. Prediction One [cited 22 Mar 2024]. https://predictionone.sony.biz/.
Nathalie J, Mohak S. Evaluating learning algorithms: a classification perspective. Cambridge: Cambridge University Press; 2011.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350: g7594.
Kasi PM. Thrombotic thrombocytopenic purpura and gemcitabine. Case Rep Oncol. 2011;4(1):143–8.
Fogli S, Danesi R, De Braud F, De Pas T, Curigliano G, Giovannetti G, et al. Drug distribution and pharmacokinetic/pharmacodynamic relationship of paclitaxel and gemcitabine in patients with non-small-cell lung cancer. Ann Oncol. 2001;12(11):1553–9.
Coleman JA, Yip W, Wong NC, Sjoberg DD, Bochner BH, Dalbagni G, et al. Multicenter phase II clinical trial of gemcitabine and cisplatin as neoadjuvant chemotherapy for patients with high-grade upper tract urothelial carcinoma. J Clin Oncol. 2023;41(8):1618–25.
Song B, Zhou S, Li C, Zheng H, Zhang X, Jin X, et al. A prediction model for chemotherapy-induced thrombocytopenia based on real-world data and a close relationship between AST/ALT ratio and platelet count in patients with solid tumors. Int J Gen Med. 2022;15:8003–15.
Suzuki Y, Kanazawa K, Kanai R, Tomita H, Saito M, Watanabe N, et al. A case of primary lung squamous cell carcinoma mimicking malignant mesothelioma producing granulocyte colony stimulating factor with chemotherapy (cisplatin and gemcitabine)-associated thrombotic thrombocytopenic purpura (TTP); an autopsy case report. Lung Cancer. 2019;136:105–8.
Nishijima Y, Hirata H, Himeno A, Kida H, Matsumoto M, Takahashi R, et al. Drug-induced thrombotic thrombocytopenic purpura successfully treated with recombinant human soluble thrombomodulin. Intern Med. 2013;52(10):1111–4.
Alabiso I, Baratelli C, Brizzi MP, Bitossi R, Ottone A, Tampellini M. Acute and fatal thrombocytopenic thrombotic purpura after a single dose of pemetrexed. Int J Clin Pharm. 2014;36(6):1141–3.
Thachil J. Causes of thrombotic thrombocytopenic purpura. Lancet Oncol. 2007;8(9):757–8.
Kuenen BC, Levi M, Meijers JC, van Hinsbergh VW, Berkhof J, Kakkar AK, et al. Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J Clin Oncol. 2003;21(11):2192–8.
Zupancic M, Shah PC, Shah-Khan F. Gemcitabine-associated thrombotic thrombocytopenic purpura. Lancet Oncol. 2007;8(7):634–41.
Acknowledgements
The authors would like to thank Editage (https://www.editage.com/) for editing and reviewing this manuscript for English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shigeki Yamada has received honoraria from Nippon Shinyaku Co., Ltd, Pfizer Japan Inc., and Takeda Pharmaceutical Co., Ltd. Tomohiro Mizuno is an editorial board member of Clinical Drug Investigation. Noriaki Matsumoto, Yosuke Ando, Koki Kato, Masanori Nakanishi, Tsuyoshi Nakai, Jeannie K. Lee, Yoshitaka Kameya, Wataru Nakamura, Kiyoshi Takahara, and Ryoichi Shiroki declare no conflicts of interest regarding the subject of this study.
Ethics approval
This study was approved by the Ethics Board of Fujita Health University Hospital (Ethics Committee approval number: HM23-027; date of approval 9 May 2023) and was conducted in accordance with the appropriate guidelines.
Consent for publication
Not applicable.
Consent to participants
An opt-out approach was used to obtain informed consent according to the guidelines of the Fujita Health University Hospital Ethics Board.
Code availability
Not applicable.
Author contributions
NM contributed to the study conception and design and drafted the manuscript. NM, KK, MN, and TN analyzed and interpreted the data. YA, JKL, YK, WN, KT, RS, and SY contributed to the study design and reviewed the manuscript. TM contributed to the study conception and design, drafted the manuscript, and supervised the execution of the study.
Data availability statement
Data archiving is not mandated but data will be made available on reasonable request.
Funding
This work was supported by the JSPS KAKENHI (grant number 21K06696) and the Research Center for Pathogenesis of Intractable Diseases, Research Institute of Meijo University, Japan.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsumoto, N., Mizuno, T., Ando, Y. et al. Prediction Model for Severe Thrombocytopenia Induced by Gemcitabine Plus Cisplatin Combination Therapy in Patients with Urothelial Cancer. Clin Drug Investig 44, 357–366 (2024). https://doi.org/10.1007/s40261-024-01361-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-024-01361-3