Abstract
Purpose
Patients affected by heart failure with reduced ejection fraction (HFrEF) receive clinical and functional beneficial effects from treatment with sacubitril/valsartan. However previous studies have shown that patients with an implantable cardioverter defibrillator (ICD) could obtain even greater benefit, but only make up a only a small proportion of patients. In the current study we evaluated the effect of sacubitril/valsartan in patients with an ICD.
Methods
Thirty-five outpatients with HFrEF (aged 60 ± 11 years, 28 were males), on optimal medical therapy were studied. All patients received an ICD at least 6 months before enrollment or were non-responders to ICD plus resynchronization (CRT-D). An open-label sacubitril/valsartan treatment was established at the maximum tolerated dose. Clinical assessment, 6-min walk test (6MWT) and echocardiography, were performed during follow-up at 90, 180, and 360 days. Quality of life score and perceived fatigue on exercise were assessed.
Results
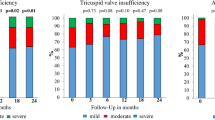
Clinical conditions dramatically improved in most patients, especially within the first 6 months of therapy (76 % were in NYHA-I and 24 % in NYHA-II at the end of study vs 71 % NYHA-II and 29 % NYHA III at enrollment, p < 0.001). Quality of life and exercise performance significantly improved according to N-terminal pro-brain natriuretic peptide (NT-proBNP) serum levels lowering. Walking distance at 6MWT increased from 274 ± 97 to 389 ± 53 m and walking speed from 0.74 ± 0.27 to 1.07 ± 0.15 m/s (p < 0.001), while oxygen saturation did not differ significantly (from 90 ± 1 % to 91 ± 2 %). More gradual was left ventricular reverse remodeling. Ejection fraction improved mildly (+ 5 points %, p < 0.001). Global longitudinal strain and diastolic function were also assessed over time.
Conclusion
Sacubitril/valsartan therapy for HFrEF may lead to significant clinical and functional improvements even in patients with ICD at greater arrhythmic risk. Clinical improvement is obtained within the first 6 months of treatment while reverse remodeling needs more time.



Similar content being viewed by others
References
Blood AJ, Fraiche AM, Eapen ZJ. Is an admission for decompensated heart failure inevitable? Prog Cardiovasc Dis. 2017;60:171–7.
Camplain R, Kucharska-Newton A, Keyserling TC, Layton JB, Loehr L, Heiss G. incidence of heart failure observed in emergency departments, ambulatory clinics, and hospitals. Am J Cardiol. 2018;11:1328–35.
Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD, et al. 10-year risk equations for incident heart failure in the general population. J Am Coll Cardiol. 2019;73:2388–97.
Ziaeiana B, Fonarowc GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379–85.
Zacà V. Sacubitril/valsartan or an implantable cardioverter-defibrillator in heart failure with reduced ejection fraction patients: a cost-effectiveness analysis. J Cardiovasc Med (Hagerstown). 2018;19(10):597–605.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. PARADIGM-HF investigators and committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004.
Ahn R, Prasad V. Do Limitations in the design of PARADIGM-HF justify the slow real world uptake of Sacubitril/Valsartan (Entresto)? Cardiovasc Drugs Ther. 2018;32(6):633–35 (Erratum in: Cardiovasc Drugs Ther. 2018;32(6):637).
Tsutsui H, Momomura S, Saito Y, Ito H, Yamamoto K, Ohishi T, et al. Angiotensin receptor neprilysin inhibitor in Japanese patients with heart failure and reduced ejection fraction—baseline characteristics and treatment of PARALLEL-HF Trial. Circ J. 2018;82:2575–83.
Yee D, Novak E, Platts A, Nassif ME, LaRue SJ, Vader JM. Comparison of the Kansas City Cardiomyopathy Questionnaire and Minnesota Living With Heart Failure Questionnaire in predicting heart failure outcomes. Am J Cardiol. 2019;123:807–12.
Wahr DW, Wang YS, Schiller NB. Left ventricular volumes determined by two-dimensional echocardiography in a normal adult population. J Am Coll Cardiol. 1983;1:863–8.
Dattilo G, Imbalzano E, Lamari A, et al. Ischemic heart disease and early diagnosis. Study on the predictive value of 2D strain. Int J Cardiol. 2016;215:150–6.
Dattilo G, Imbalzano E, Casale M, et al. Psoriasis and cardiovascular risk: correlation between psoriasis and cardiovascular functional indices. Angiology. 2018;69(1):31–7.
de Gregorio C, Dattilo G, Casale M, et al. Left atrial morphology, size and function in patients with transthyretin cardiac amyloidosis and primary hypertrophic cardiomyopathy—comparative strain imaging study. Circ J. 2016;80(8):1830–7.
Williams LK, Urbano-Moral JA, Rowin EJ, Jamorski M, Bruchal-Garbicz B, Carasso S, et al. Left atrial morphology, size and function in patients with transthyretin cardiac amyloidosis and primary hypertrophic cardiomyopathy—comparative strain imaging study. Circ J. 2016;80(8):1830–7.
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117.
de Gregorio C. Physical training and cardiac rehabilitation in heart failure patients. Adv Exp Med Biol. 2018;1067:161–81.
Borg E, Borg G. A comparison of AME and CR100 for scaling perceived exertion. Acta Psychol (Amst). 2002;109:157–75.
Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7.
Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68:2425–36.
Vardeny O, Claggett B, Kachadourian J, Pearson SM, Desai AS, Packer M, et al. Incidence, predictors, and outcomes associated with hypotensive episodes among heart failure patients receiving Sacubitril/Valsartan or Enalapril: The PARADIGM-HF Trial (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin-Converting Enzyme Inhibitor to determine impact on global mortality and morbidity in heart failure). Circ Heart Fail. 2018;11(4):e004745.
Morrow DA, Velazquez EJ, DeVore AD, Desai AS, Duffy CI, Ambrosy AP, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to Sacubitril/Valsartan or Enalapril in the PIONEER-HF Trial. Circulation. 2019;139:2285–8.
Moliner-Abós C, Rivas-Lasarte M, Pamies Besora J, Fluvià-Brugues P, Solé-González E, Mirabet S, et al. Sacubitril/Valsartan in real-life practice: experience in patients with advanced heart failure and systematic review. Cardiovasc Drugs Ther. 2019;33(3):307–14.
Nougué H, Pezel T, Picard F, Sadoune M, Arrigo M, Beauvais F, et al. Effects of Sacubitril/Valsartan on Neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur J Heart Fail. 2019;21:598–605.
Buggey J, Mentz RJ, Devore AD, Velazquez EJ. Angiotensin receptor neprilysin inhibition in heart failure: mechanistic action and clinical impact. J Cardiac Fail. 2015;21:741–50.
Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, et al. Effects of Sacubitril/Valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF Trial. JAMA Cardiol. 2018;3:498–505.
Aronow WS, Shamliyan TA. Benefits and harms of sacubitril in adults with heart failure and reduced left ventricular ejection fraction. Am J Cardiol. 2017;120:1166–70.
Martens P, Beliën H, Dupont M, Vandervoort P, Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther. 2018;36:e12435.
Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al. Association of change in N-terminal pro-B-type Natriuretic Peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019. https://doi.org/10.1001/jama.2019.12821 ((in press)).
Hsiao FC, Wang CL, Chang PC, Lu YY, Huang CY, Chu PH. Angiotensin receptor neprilysin inhibitor for patients with heart failure and reduced ejection fraction: real-world experience from Taiwan. J Cardiovasc Pharmacol Ther. 2019:1074248419872958 (in press).
Čelutkienė J, Plymen CM, Flachskampf FA, de Boer RA, Grapsa J, Manka R, et al. Innovative imaging methods in heart failure: a shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:1615–33.
De Vecchis R, Paccone A, Di Maio M. Sacubitril/Valsartan therapy for 14 months induces a marked improvement of global longitudinal strain in patients with chronic heart failure: a retrospective cohort study. Cardiol Res. 2019;10:293–302.
Maslov MY, Foianini S, Orlov MV, Januzzi JL, Lovich MA. A novel paradigm for sacubitril/valsartan: beta-endorphin elevation as a contributor to exercise tolerance improvement in rats with preexisting heart failure induced by pressure overload. J Cardiac Fail. 2018;24:773–82.
Casale M, Mezzetti M, Tulino V, et al. Therapy of cardiac arrhythmias in children: an emerging role of electroanatomical mapping systems. Curr Vasc Pharmacol. 2018;16(6):528–33. https://doi.org/10.2174/1570161115666170705155542.
Casale M; Mezzetti M, Gigliotti De Fazio M et al. Usefulness of Sacubitril/Valsartan in reduction of atrial fibrillation burden in a patient with ICD delivering inappropriate therapies. A new possibility? Cor Vasa 2020;62(3):336–39. https://doi.org/10.33678/cor.2020.004
Pocock SJ, Assmann SF, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no external funding.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Conceptualization, GD and CG; methodology, GD and CG; formal analysis, MC and GD; investigation, GL, VV, CM; data curation, MC and MC; writing—original draft preparation, MC and MC; writing—review and editing, MC and MC; visualization, SSS, FL and NK; supervision, FL; project administration, GD and CG. All authors have read and agreed to the published version of the manuscript.
Rights and permissions
About this article
Cite this article
Casale, M., Correale, M., Laterra, G. et al. Effects of Sacubitril/Valsartan in Patients with High Arrhythmic Risk and an ICD: A Longitudinal Study. Clin Drug Investig 41, 169–176 (2021). https://doi.org/10.1007/s40261-020-00995-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00995-3