Abstract
Background
Enoxaparin is a low-molecular weight heparin (LMWH) commonly used for treatment of venous thromboembolism and acute coronary syndromes. The recommended dose for these conditions is weight-based (1 mg/kg) and doesn’t require dose-capping. However, previous studies have shown that in those with a body mass index (BMI) > 40 kg/m2, this dose results in supratherapeutic levels.
Objective
This study investigated enoxaparin dosing in morbidly obese patients with a goal of identifying a dose with the greatest chance of producing favorable anti-factor Xa (anti-Xa) levels.
Methods
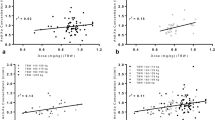
This retrospective cohort study by electronic chart review was used to record data of patients who received enoxaparin with anti-Xa level monitoring between 2012 and 2017. The primary outcome was the enoxaparin dose that results in a therapeutic anti-Xa level (0.5–1.0 IU/mL) among three BMI groups. Secondary outcomes were bleeding and thromboembolic events.
Results
Two hundred forty-one patients were included in the study, and 132 achieved a therapeutic dose. For those with a BMI of 40–50 kg/m2, the median therapeutic dose was 0.97 mg/kg every 12 h. In subjects with a BMI of 50–60 kg/m2, the median therapeutic dose was 0.70 mg/kg. Finally, the median therapeutic dose for subjects with a BMI over 60 kg/m2 was 0.71 mg/kg. In all three groups, 53–65% of patients had a supratherapeutic anti-Xa level while less than 10% had a subtherapeutic level. Relatively few patients (4.1%) experienced major bleeding and only one thromboembolic event was reported.
Conclusion
Standard dosing of enoxaparin in morbidly obese patients will most likely lead to supratherapeutic anti-Xa levels and thus further investigation is warranted to better determine appropriate dosing.

Similar content being viewed by others
References
World Health Organization. Obesity and overweight. Updated February 2018. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 19 Apr 2019.
Centers for Disease Control and Prevention. Adult obesity facts. Updated August 2018. https://www.cdc.gov/obesity/data/adult.html. Accessed 19 Apr 2019.
Lovenox [package insert]. Bridgewater: Sanofi-Aventis U.S.; 2013.
Abernethy DR, Greenblatt DJ. Pharmacokinetics of drugs in obesity. Clin Pharmacokinet. 1982;7(2):108–24. https://doi.org/10.2165/00003088-198207020-00002.
Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. https://doi.org/10.2165/11318100-000000000-00000.
Freeman AL, Pendleton RC, Rondina MT. Prevention of venous thromboembolism in obesity. Expert Rev Cardiovasc Ther. 2010;8(12):1711–21. https://doi.org/10.1586/erc.10.160.
Spinler SA, Ou FS, Roe MT, et al. Weight-based dosing of enoxaparin in obese patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE initiative. Pharmacotherapy. 2009;29(6):631–8. https://doi.org/10.1592/phco.29.6.631.
Thompson-Moore NR, Wanat MA, Putney DR, Liebl PH, Chandler WL, Muntz JE. Evaluation and pharmacokinetics of treatment dose enoxaparin in hospitalized patients with morbid obesity. Clin Appl Thromb Hemost. 2015;21(6):513–20. https://doi.org/10.1177/1076029614568713.
Deal EN, Hollands JM, Riney JN, Skrupky LP, Smith JR, Reichley RM. Evaluation of therapeutic anticoagulation with enoxaparin and associated anti-Xa monitoring in patients with morbid obesity: a case series. J Thromb Thrombolysis. 2011;32:188–94. https://doi.org/10.1007/s11239-011-0584-7.
Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e24S–43S. https://doi.org/10.1378/chest.11-2291.
Monagle P, Michelson AD, Bovill E, et al. Antithrombotic therapy in children. Chest. 2001;119:344S–70S.
Nutescu EA, Spinler SA, Wittkowsky A, Dager WE. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother. 2009;43(6):1064–83. https://doi.org/10.1345/aph.1L194.
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4.
Bazinet A, Almanric K, Brunet C, et al. Dosage of enoxaparin among obese and renal impairment patients. Thromb Res. 2005;116(1):41–50.
Lalama JT, Feeney ME, Vandiver JW, Beavers KD, Walter LN, McClintic JR. Assessing an enoxaparin dosing protocol in morbidly obese patients. J Thromb Thrombolysis. 2015;39(4):516–21. https://doi.org/10.1007/s11239-014-1117-y.
Curry MA, LaFollette JA, Alexander BR, et al. Evaluation of treatment-dose enoxaparin in acutely ill morbidly obese patients at an academic medical center: a randomized clinical trial. Ann Pharmacother. 2018. https://doi.org/10.1177/1060028018821149.
Maclachlan KH, Stevens HP, Tran HA, et al. Weight-based enoxaparin for venous thromboembolism in obesity gives similar anti-Xa levels to patients < 100 kg, with no increase in major bleeding. Semin Thromb Hemost. 2019;45(1):94–9. https://doi.org/10.1055/s-0038-1677019.
Czupryn MJ, Exline C. Dosing of enoxaparin in morbidly obese patients: a retrospective cohort. Hosp Pharm. 2018;53(5):331–7. https://doi.org/10.1177/0018578718757518.
Acknowledgements
The authors wish to acknowledge the contribution of the Texas Tech University Health Sciences Center Clinical Research Institute for their assistance with this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received in relation to the conduct of this study.
Conflict of interest
Young R. Lee, Peter J. Palmere, Caitlin E. Burton, and Taylor M. Benavides declare that they have no conflict of interest.
Informed Consent
For this type of study, formal consent is not required.
Ethics Approval
Our study was reviewed and approved by the Texas Tech University Health Sciences Center Institutional Review Board.
Rights and permissions
About this article
Cite this article
Lee, Y.R., Palmere, P.J., Burton, C.E. et al. Stratifying Therapeutic Enoxaparin Dose in Morbidly Obese Patients by BMI Class: A Retrospective Cohort Study. Clin Drug Investig 40, 33–40 (2020). https://doi.org/10.1007/s40261-019-00855-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00855-9