Abstract
Conventional vaccines have been widely studied, along with their risk of causing allergic reactions. These generally consist of mild local reactions and only rarely severe anaphylaxis. Although all the current COVID-19 vaccines marketed in Europe have been shown to be safe overall in the general population, early post-marketing evidence has shown that mRNA-based vaccines using novel platforms (i.e., lipid nanoparticles) were associated with an increased risk of severe allergic reactions as compared to conventional vaccines. In this paper we performed an updated literature review on frequency, risk factors, and underlying mechanisms of COVID-19 vaccine-related allergies by searching MEDLINE and Google Scholar databases. We also conducted a qualitative search on VigiBase and EudraVigilance databases to identify reports of “Hypersensitivity” and “Anaphylactic reaction” potentially related to COVID-19 vaccines (Comirnaty, Spikevax, Vaxzevria and COVID-19 Janssen Vaccine), and in EudraVigilance to estimate the reporting rates of “Anaphylactic reaction” and “Anaphylactic shock” after COVID-19 vaccination in the European population. We also summarized the scientific societies’ and regulatory agencies’ recommendations for prevention and management of COVID-19 vaccine-related allergic reactions, especially in those with a history of allergy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Serious allergic reactions observed after the receipt of an mRNA vaccine are rare. |
Reporting rate of serious allergic reactions for viral vector COVID-19 vaccines seems to be similar to that for COVID-19 mRNA vaccines. |
The underlying mechanism of COVID-19 vaccine-related allergies have not yet been fully elucidated. |
A risk stratification assessment for the development of allergic reactions should be conducted before the receipt of a vaccine against COVID-19. |
1 Introduction
Since December 2020, four COVID-19 vaccines have been marketed under conditional approval in the European Union (EU): the mRNA-based vaccines, Comirnaty (developed by Pfizer-BioNTech) and Spikevax (developed by Moderna), and the viral vector vaccines, Vaxzevria (ex-COVID-19 Vaccine AstraZeneca) and COVID-19 Janssen Vaccine (developed by Johnson & Johnson) [1]. On 20th December 2021, European Medicine Agency (EMA) also recommended granting a conditional to the Nuvaxovid vaccine (developed by Novavax) to prevent COVID-19 in people aged ≥ 18 years.
Conventional vaccines have been widely studied, along with their risk of causing allergic reactions. These generally consist of mild local reactions (e.g., pain, redness, swelling at the injection site), and very rarely severe reactions such as anaphylaxis [2, 3]. Although all the above-mentioned COVID-19 vaccines have been shown to be safe in the general population, early post-marketing evidence showed that mRNA-based vaccines were associated with an increased risk of severe allergic reactions as compared to conventional vaccines (more than 10 per million doses vs 1.4 per million doses, respectively) [4, 5]. However, the estimated severe allergic reaction reporting rate has been decreasing since the beginning of the vaccination campaign. As mRNA-based vaccines rely on innovative technologies (i.e., lipid nanoparticles), it is essential to carry out an in-depth evaluation of their safety profile and compare the risks of severe allergic reactions across available COVID-19 vaccines. Even if cumulated evidence so far indicates that benefits of vaccination against COVID-19 outweigh the potential risks of severe allergic reactions, the fear of allergic reactions can still create uncertainty among the population, in particular those people with a history of allergy that represent contraindication to the COVID-19 vaccine administration. In addition, with new marketed vaccines, especially initially, there are more immunization stress-related responses (ISRR) [6]. Risk factors and the exact underlying mechanisms of COVID-19 vaccine allergic reactions have not yet been completely elucidated, which limits the comparative assessment of severe allergic reactions across different vaccines and ultimately the risk minimization measures for preventing COVID-19 vaccine-related allergic reactions. The aim of this review is to summarize the currently available evidence on frequency, risk factors, and underlying mechanisms of allergic reactions related to different COVID-19 vaccines and also on current recommendations for prevention and management of COVID-19 vaccine-allergic reactions, especially in those with a history of allergy.
2 Frequency of Allergic Reactions to COVID-19 Vaccines
2.1 Evidence from Post-marketing Observational Studies
People with a previous history of allergy were excluded from the pivotal trials of all COVID-19 vaccines currently approved by the EMA. As such, evidence on potential risks of allergy related to COVID-19 vaccines from a real-world setting is needed. Therefore, a literature search was carried out up to March 15, 2022, in MEDLINE and Google Scholar databases to identify observational studies and case series concerning COVID-19 vaccine-related allergies. Although no language restrictions were initially applied in the search strategy, only articles in English were considered. Most of the identified studies were from the USA and concerned mRNA COVID-19 vaccines.
Those studies consisted of analysis of spontaneous reporting databases as well as prospective/retrospective cohort studies.
According to the Centers for Disease Control and Prevention (CDC) report of the US Vaccine Adverse Event Reporting System (VAERS) database, in the period between December 14, 2020, and January 18, 2021, 47 cases of anaphylaxis from more than 9 million administered doses of Comirnaty vaccine (4.7 cases per million doses), and 19 cases for more than 7 million doses of the Spikevax vaccine (2.5 cases per million doses) were reported [7]. A subsequent disproportionality analysis of data collected between December 1, 2020, and March 5, 2021 from VAERS showed no statistically significant association of anaphylactic reactions and mRNA vaccines (proportional reporting ratio: 1.26; 95% CI 0.18–9.05), applying conventional cut-off for signal detection using spontaneous reporting databases [8, 9]. Similarly, in VAERS from December 14, 2020 to September 30, 2021, anaphylaxis rates of 5 per million doses for Comirnaty and 3 per million doses for Spikevax were documented [10]. In addition, this study estimated a rate of 9 cases per million doses for the COVID-19 Janssen vaccine [10]. With the inclusion of data from the v-safe, the US voluntary smartphone-based system for the collection of adverse events, in addition to those of the VAERS database, the reporting rate of anaphylaxis between December 14, 2020 and June 14, 2021 was estimated to be slightly higher: 5.8 per million doses administered of Comirnaty and 5.1 per million doses administered of Spikevax [11]. These results are consistent with the finding in the study of Klein and colleagues [12]: in the period December 14, 2020 to June 26, 2021, according to the Brighton case definition for anaphylaxis [13], of more than 11 million administered doses in the US, a reporting rate of 4.8 and 5.1 cases per million doses administered was identified for Comirnaty and Spikevax, respectively. There were similar findings in a Korean study evaluating anaphylaxis cases between February 26, 2021 and October 31, 2021 using the Brighton case definition for levels 1, 2, or 3 and resulting in 6.6 cases of anaphylaxis per million doses administered of Comirnaty and 4.3 cases per million doses administered of Spikevax [14]. In the same study, 4.2 and 14.1 cases of anaphylaxis per million doses following the receipt of Vaxzevria and COVID-19 Janssen vaccine were reported [14].
Compared to data from VAERS, the Ontario’s vaccine safety surveillance system reported a higher rate for anaphylaxis. Between December 13, 2020, and March 6, 2021, of 890,604 doses of mRNA vaccines administered, 15 cases of anaphylaxis meeting the Brighton Collaboration case definition criteria for levels 1, 2, or 3 were reported (17 per million doses administered). The reporting rate for anaphylaxis was higher following the vaccination with Comirnaty as compared to Spikevax (19 vs 7 per million doses) [15]. The Japanese Pharmaceutical and Medical Devices Agency (PMDA) in the period February to March 2021 found a reporting rate of 81 per million doses administered for mRNA-based vaccines; however, both reports of anaphylaxis and anaphylactoid symptoms were included in this analysis [16].
In addition to data on severe allergic reactions collected from the spontaneous reporting systems, several cohort studies measured the frequency of COVID-19 vaccine-related allergic reactions.
An Israelian prospective cohort study conducted in the period December 27, 2020–February 22, 2021, found that the rate of allergic reactions to Comirnaty was higher among people with a history of allergy and even more for those with a history of high-risk allergies [17]. Of 429 high-risk patients included, allergic reactions to Comirnaty vaccine were observed in 1.4% and 1.8% after the first and second dose, respectively; however, anaphylactic reactions were observed only following the administration of the first dose of vaccine at a percentage of 0.7%. Similarly, a US prospective study collected data on acute allergic reaction from 64,900 Mass General Brigham employees receiving a first dose of a mRNA-based COVID-19 vaccine in the period between December 16, 2020, and February 18, 2021 [18]. Overall, around 1300 (2.0%) participants reported an allergic reaction, generally mild, such as itching, rash, hives, swelling, and/or respiratory symptoms. Anaphylaxis, confirmed by either the Brighton Collaboration case definition [13] or the National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network (NIAID/FAAN clinical criteria) [19], was observed in 7 (0.03%) subjects following the receipt of Comirnaty and in 9 (0.02%) subjects following that of Spikevax [18]. Another observational study of 28,544 employees and health care providers receiving a first dose of a mRNA-based vaccine in the period between December 15, 2020, and March 8, 2021, reported 1253 vaccine-related adverse reactions. For 113 subjects, the experts required further evaluation before receiving the second dose, since three persons reported symptoms that were judged to be consistent with anaphylaxis [20].
There is currently limited evidence on rates of allergic reactions following the immunization with the Vaxzevria and the COVID-19 Janssen vaccines. In a prospective Korean study on a population of more than 5500 vaccinees [21], those receiving Vaxzevria (N = 5589) reported a significantly higher number of adverse reactions related to allergy, compared to those receiving Comirnaty (N = 227). Specifically, 5217 and 222 subjects reported at least one adverse reaction following the first dose of Vaxzevria and Comirnaty, respectively, including angioedema (19.1% vs 4.3%), tongue edema (18.0% vs 3.6%), foreign body sensation in the throat (24.3% vs 9.7%), wheezing (6.4% vs 0.7%), urticaria (5.8% vs 0.7%) and skin rash (5.7% vs 1.8%). A study conducted in Saudi Arabia which included 7768 Vaxzevria vaccinees and 5100 receiving Comirnaty, no case of anaphylaxis was reported. However, authors reported that mild/moderate allergic reactions occurred at a frequency of 4.5% for the Vaxzevria vaccine and 7.2% for Comirnaty [22].
The data reported in this section show that there is high variability among the reporting rate of allergic reactions, such as anaphylaxis. This may be related to different point in time of data collection, different source of information (passive spontaneous reporting surveillance system vs observational studies), different settings and countries, or even to the different application of the diagnostic criteria for the identification of serious allergic reactions, such as anaphylaxis (Brighton Collaboration case definition vs other criteria). Since the priority at the beginning of the pandemic was to ensure the safety of the vaccines by identifying the life-threatening adverse reactions, it might be the case that non-severe allergic reactions were generally under-reported in spontaneous reporting systems. Thus, we could not provide the accurate estimate of the rate of overall allergic reactions and of anaphylactic reactions, specifically since this would have required an exact categorization of serious/non-serious allergic reactions from different studies, which was not always possible.
2.2 Analysis of Spontaneous Adverse Reactions Reporting System Databases: Vigibase and EudraVigilance
To provide an updated overview of allergic events following anti-COVID-19 immunization, a descriptive analysis of two international spontaneous reporting databases, Vigibase and EudraVigilance, collecting adverse drug reactions (ADRs) and adverse events following immunization (AEFIs) was performed. Up to December 14, 2021, a total of 8200,642,671 COVID-19 vaccine doses have been administered worldwide [23]. On the same date, we conducted a qualitative search using VigiLyze® [24], a tool that provides access to Individual Case Safety Reports (ICSRs) collected in VigiBase, the World Health Organization (WHO) pharmacovigilance database developed and maintained by Uppsala Monitoring Centre containing ICSRs from more than 149 member countries.
We searched for reports including a Medical Dictionary for Regulatory Activities (MedDRA) Preferred Term (PT) belonging to the Standardised MedDRA Queries (SMQ) “Hypersensitivity” selecting the option “Narrow” and filtering by drug trade name (Comirnaty; Spikevax; Vaxzevria and COVID-19 Janssen Vaccine). Then, we used the same search strategy to retrieve reports including a MedDRA PT term belonging to the SMQ “Anaphylactic reaction”. Reports could not be differentiated by first and second doses. According to the Introductory Guide for SMQ (version 24.1), “Hypersensitivity” can be intended as “a large number of conditions related to an exaggerated response of the body to a foreign agent”, while the term “Anaphylactic reaction” is referring to “an acute systemic reaction characterized by pruritus, generalized flush, urticaria, respiratory distress and vascular collapse” [25].
By December 14, 2021 (last data drawn), there was a total of 615,488 ICSRs reporting Comirnaty as suspected vaccine, 174,038 ICSRs reporting Vaxzevria, 114,187 ICSRs reporting COVID-19 Janssen Vaccine and 26,731 ICSRs reporting Spikevax. Concerning the SMQ “Hypersensitivity”, 50,106 of 615,488 ICSRs (8.1%) and 3029 of 26,731 ICSRs (11.3%) were identified for the mRNA-based vaccines Comirnaty and Spikevax, respectively; 10,455 of 174,038 ICSRs (6.0%) and 8565 of 114,187 ICSRs (7.5%) were identified for the viral vector vaccines Vaxzevria and COVID-19 Janssen Vaccine, respectively. As for the SMQ “Anaphylactic reaction”, following the mRNA-based vaccines, 3421 of 615,488 ICSRs (0.6%) and 78 of 26,731 ICSRs (0.3%) were identified for Comirnaty and Spikevax, respectively; 554 of 174,038 ICSRs (0.3%) and 548 of 114,187 ICSRs (0.5%) were identified for Vaxzevria and COVID-19 Janssen Vaccine, respectively.
For both the SMQ “Hypersensitivity” and “Anaphylactic Reaction”, the frequency of reports was higher in females than males, regardless of the type of vaccine. Almost all the reports for both the SMQ “Hypersensitivity” and “Anaphylactic Reaction’’ concerned adults (aged ≥18 years). This result is expected given that EMA recommended the use of the two mRNA COVID-19 vaccines in children aged 12–15 just five months after the start of the vaccination campaign in the adult population; moreover, only Comirnaty has been authorized in children aged 5–11 years by the end of November 2021 in the EU [26]. Concerning pediatric population aged below 18, a total number of 1969 ICSRs were retrieved for the SMQ “Hypersensitivity”, including 1781 (90.5%) Comirnaty ICSRs, 160 (8.1%) Spikevax ICSRs, 9 (0.4%) Vaxzevria ICSRs and 19 (1.0%) Janssen ICSRs. A total number of 119 ICSRs were retrieved for the SMQ “Anaphylactic reaction”, including 113 (95.0%) Comirnaty ICSRs, 1 (0.8%) Spikevax ICSRs, 2 (1.7%) Vaxzevria ICSRs and 3 (2.5%) Janssen ICSRs. Concerning Vaxzevria and Janssen Vaccine, pediatric ICSRs are likely due to administration errors or errors in indicating the age of vaccinee in the ICSR.
Concerning the SMQ “Hypersensitivity”, most reports were classified as not serious. As for serious reports, a similar rate was observed for Comirnaty (22.4%), Spikevax (24.8%) and Vaxzevria (23.1%), which suggests a comparable safety profile for these three vaccines. In contrast, a lower percentage (14.3%) of COVID-19 Janssen Vaccine-related reports was classified as serious.
On the other hand, most of the reports identified for the SMQ “Anaphylactic reaction” were classified as serious (68.9%).
All the above-mentioned data are reported in Table 1.
We analyzed Vigibase, as collecting a high number of reports from many countries outside Europe; however, the reporting rate in this database could not be calculated as the vaccine-specific number of administered doses from all different countries contributing reports to Vigibase is not available.
To better compare the allergic reaction reporting across vaccines, we estimated the reporting rate of COVID-19 vaccine-related allergic reactions, analyzing publicly available reports from EudraVigilance (EV), the spontaneous reporting ADRs/AEFIs database that is maintained by the EMA [27]. On December 14, 2021, the search included the MedDRA PTs “Anaphylactic reaction” and “Anaphylactic shock” following COVID-19 vaccination. To calculate the reporting rate, the analysis was restricted to ICSRs from the European Economic Area (EEA) only, for which the number of administrated vaccine doses could be retrieved during the same observation period.
According to the last data drawn (December 11, 2021), a total of 1975 reports of anaphylactic shock and/or anaphylactic reaction was identified following mRNA-based vaccines, yielding a reporting rate of 3 per million doses administered for Comirnaty, and 2 per million doses administered for Spikevax. Concerning viral vector vaccines, a reporting rate of 3 per million administered doses for Vaxzevria, and 2 per million administered doses for COVID-19 Janssen Vaccine was calculated. A similar reporting rate of anaphylactic shock and/or anaphylactic reaction has been reported for mRNA and viral vector COVID-19 vaccines.
3 Risk Factors of Allergic Reactions to COVID-19 Vaccines: Data from Real-World Setting
According to a large body of clinical evidence, COVID-19 vaccines have been documented as having an overall high degree of efficacy and safety. However, severe allergic reactions have been reported since the beginning of the vaccination campaign [28, 29], which suggests a higher rate of anaphylaxis due to the COVD-19 vaccine in comparison with other marketed vaccines. The identification of risk factors for allergic reactions as well as the safety of these vaccines in categories of persons at higher risk of developing allergic reactions have to be carefully assessed. Several post-marketing observational studies reported a higher prevalence of allergic reactions in patients self-reporting previous (mostly drug-related) anaphylaxis [16, 30]. The reported cases of anaphylaxis for both mRNA vaccines occurred most often after the first dose (82%), in women (> 90%), and in subjects with a history of allergic reactions (79%) [7]. These results were confirmed in a prospective Israeli study, which observed a slightly higher rate of allergic reaction (6%) and anaphylaxis (0.7%) in persons at high-risk of allergic reactions, such as those with prior anaphylactic reaction to any drug or vaccine, multiple drug allergies, or in general multiple allergies [17]. Subjects who reported anaphylaxis were aged between 40 and 50 years, which is typically the mean age of patients with drug allergy [31]. All these studies reported a striking prevalence of COVID-19 vaccines-related allergic reactions in females [12, 16,17,18, 30]. These data are consistent with a higher risk of drug allergy and drug-induced anaphylaxis in women, which emerges particularly after puberty [32].
The possible connection between an allergic response to the COVID-19 vaccines and female sex may be related to diverse effects of sexual hormones on the immune system. Estrogen may induce a TH2 response, promote mast cell degranulation and, in murine models, increase endothelial nitric oxide synthase activity, enhancing the severity of anaphylaxis. On the other hand, testosterone and progesterone reduce TH2 responses suppressing histamine release from mast cells [33,34,35,36]. Moreover, a more frequent exposure to cosmetics and drugs may account for the increased risk of allergic reactions among females. Acute and chronic stress can also induce mast cell degranulation through the release of corticotropin-releasing hormones, neurotensin and substance P of neuronal origin [37,38,39]. The use of non-steroidal anti-inflammatory drugs just before or after the vaccination has to be carefully investigated as these drugs can themselves be responsible for anaphylaxis or are potential co-factors [37, 40]. No reaction to COVID-19 vaccination has been related to physical exercise, which is a risk factor for anaphylaxis.
Another crucial issue is the safety of the COVID-19 vaccination in at-risk populations. An association has been reported between severe asthma and anaphylaxis, particularly in women [41].
In a large cohort of patients with severe asthma, according to the European Respiratory Society (ERS) and American Thoracic Society (ATS) criteria, undergoing the vaccination in a specialist allergy-pneumology setting [42], only mild reactions, mainly local, were observed after the administration of both doses. It is worth noting that in these patients, asthma remained under control between the two doses and quality of life improved [43]. According to a Spanish study, the COVID-19 vaccination, carried out in a specialized setting, was also well tolerated in patients with severe allergic conditions, such as hymenoptera, food and drug allergy [44].
Furthermore, good tolerability was reported in a population of 63 patients suffering from hereditary angioedema. After vaccination, 11 attacks were reported out of 111 administrations. All these attacks were mild and recovered within two days. Most patients did not use short-term prophylaxis [45].
All the above reported data are reported in Table 2.
4 Underlying Mechanisms of COVID-19 Vaccine-related Allergies
Like other medications, vaccines can trigger hypersensitivity reactions (HRs), which are usually rare and mild. In case of vaccine-related allergic reaction, it is difficult to ascertain whether the vaccine itself or other excipients or inactive ingredients can be the causative agent [46].
This holds true also for the approved COVID-19 vaccines, as their excipients are considered as the most probable trigger of immunoglobulin (Ig) E-mediated allergic reactions, while the vaccine antigen and the residual non-human protein are considered a less probable cause of allergy [47].
Some excipients, such as polyethylene glycol (PEG) and polysorbate (PS), belong to a family of biocompatible hydrophilic polymers, generated via polymerization of ethylene oxide [48]. PEGylation, the covalent linking of PEG to therapeutic proteins, aims to protect active ingredient from proteolytic enzymes, to reduce immunogenicity (decreased interaction of PEG-modified materials with blood components and immune cells) and to improve the water-solubility, pharmacokinetics of drugs and/or delivery vehicles. Polyethylene glycol has always been considered safe and widely used as a food and drug additive in everyday products, and its molecular weight (MW) ranges from 300 to 10,000 g/mol or higher.
4.1 Pathophysiology of Vaccine-Induced Allergic Reactions
To understand the HRs caused by the current COVID-19 vaccines, it is important to consider the potential mechanisms and pathways that may be involved (Table 3).
In general, HRs caused by vaccines can account for the classic four types of Gell and Coombs classification. Updating this classification [49], complement activation-related pseudo-allergy (CARPA) may be regarded as an independent category within “receptor-mediated” mast cell activations, usually IgE-independent, representing the main type of complement (C) activation-related HRs [50]. Clinically, CARPA is unpredictable and occasionally fatal, usually with immediate onset, at first treatment (no prior exposure to allergen), is milder or absent upon repeated exposures, with spontaneous resolution [51]. Anaphylaxis and/or anaphylactoid reactions can be mediated by either binding of the C-derived peptides C3a and C5a to their receptors on mast cells, basophils, and other myeloid cells, or by direct activation of mast cell receptors by drugs; for example, life-threatening HRs can be mediated via direct non-specific activation of the Mas-related G protein-coupled receptor X2 (MRGPRX2) on mast cells, even at first exposure, such as those containing a quaternary ammonium ion, like the Comirnaty vaccine (see Sect. 5) [52]. Notably, in MRGPRX2 activation of mast cells, the specific IgE may remain undetected, and tryptase levels may be in range, even in serious Kounis syndrome [47]. Regarding type II HRs, human IgG-mediated anaphylaxis has been described in few observations involving the parenteral administration of significant amount of allergen (i.e., protein, like chimeric, humanized, either fully human monoclonal antibodies such as infliximab or adalimumab). However, the relevance of some of these observations remains unclear, as in some cases (low) titers of drug-IgE were found [53, 54]. Intravenous injection of nanotechnology enhanced (liposomal, micellar, polymer-conjugated) and protein-based (antibodies, enzymes) drugs/vaccines can lead to infusion, or anaphylactoid reactions. The molecular mechanism underlying mild to severe allergy symptoms may differ from case to case and remains mostly uncertain. However, in many cases a major cause or contributing factor is the C system activation, through the potential mechanisms described above [48]. In addition to basophils and mast cells, neutrophils and macrophages are also considered to be relevant effector cells that can be activated via immune complex receptors (CD16, CD32, and CD64, respectively) [50, 55,56,57].
4.2 COVID-19 Vaccine Allergy: The Role of PEGs
For the first time, PEG, also known as macrogol, has been used in two of the currently marketed COVID-19 vaccines (Comirnaty and Spikevax) to stabilize and internalize the mRNA-containing lipid nanoparticles (LNP) into the cells. The mRNA COVID-19 vaccines contain no traces of food, drugs or latex, while they contain buffer constituents (tromethamine, also called trometamol, in Spikevax vaccine), and lipids (LNP). Among lipids, there are PEGylated lipids (ALC-0159), ionizable lipids (i.e., ALC-0315), neutral lipids (DSPC containing a quaternary ammonium ion), and cholesterol. Lipid nanoparticles used in Comirnaty and Spikevax vaccines may contain low levels (< 2 mol%) of 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamid and 1,2-dimyristoyl-rac-glycero-3-methoxy-[(polyethylene glycol)-2000], respectively; the type of PEG used is PEG-2000, which differs from others previously used in drugs and healthcare products both in terms of MW and co-formulation, being the stabilizing portion of a liposome [58]. Lipid nanoparticles are similar to liposomes, which have been used in the past as carriers for drugs, and further contribute to LNP stabilization by a steric mechanism [59].
An excipient structurally similar to PEG due to shared moieties (=CH2CH2 and =CH2CH2OH) is PS80, also known as Tween 80 or E 433, and it is present in more recently marketed viral vector and recombinant protein COVID-19 vaccines. Clinical cross-reactivity between PEG and PS80 has rarely been documented [48, 60, 61], but has not occurred in COVID-19 vaccine setting experience, even in case of in vitro skin test cross-reactivity [62]. Of note, in vivo and in vitro evidence of PS80 allergy is limited and isolated sensitization through PS80 seems uncommon, even rarer than through PEG [63]. PEG is the only excipient in COVID-19 vaccines that has been clearly demonstrated to cause mainly immediate HRs [64, 65], while the role of trometamol and PS80 as relevant allergens in these vaccines remains more questionable [66].
Polyethylene glycol-related HRs are more likely to occur when PEG has a MW ranging between 3350 and 6000 g/mol [48]. The higher the doses and the MW, the worse the reactions reported [67]. However, the PEG MW threshold for immediate HRs is still undetermined [64, 68].
Over the past decades, PEG has been considered non-immunogenic. However, there has been growing evidence that PEG might be more immunogenic than previously recognized, as suggested by the existence of anti-PEG antibodies in 25% of healthy blood donors [69], in 42% of patients with no history of treatment with PEGylated products [70], and the existence of anti-PEG antibodies in healthy humans who had increased exposure to PEG additives [71, 72]. Decades of clinical practice pointed out that PEGylation can result in a highly immunogenic conjugate and can cause the production of anti-drug autoantibodies (ADA) and C activation, leading to drug clearance with loss of clinical benefit or adverse HRs, as suggested by a recent murine study in which PEGylated human granulocyte colony-stimulating factor (G-CSF) elicited anti-PEG IgM immune response in a dose-dependent manner [73]. Several research groups have shown that anti-PEG antibodies were responsible for a rapid clearance of subsequent doses of PEGylated liposomes and micelles (this phenomenon was called accelerated blood clearance [ABC] phenomenon) [74,75,76]. This mechanism has been suggested for many PEGylated drugs (e.g. Pegintron®) inducing ADA in about 10% of patients and diminishing clinical benefit in about 1% of patients [77, 78].
Little is known about the prevalence of anti-PEG antibodies, even if it has been reported that as much as 72% of the general population have at least some IgG or IgM anti-PEG antibodies [70], while other authors reported higher levels in healthy people and even in those never exposed to PEGylated drugs [69, 79, 80].
Allergic reactions, even anaphylaxis, related to PEG in a variety of products have been described [47, 48, 68] and PEG is often considered a “hidden” allergen [48, 64, 81].
Besides IgE, other antibody classes may trigger HRs or amplify IgE-mediated reaction [55, 56]. Several animal studies as well as clinical observations have shown that PEGylated liposomes can activate the C system and potentially cause HRs [82,83,84]. In line with this concept, there are some studies testing basophil activation test (BAT) to the mRNA vaccines, that apparently showed that PEG-containing vaccines were able to induce positive BAT better than PEG alone [85, 86].
Irrespective of PEGylation, liposomes have the potential role to activate complement, non-specifically, and depending on their different surface structures and surface charge [57, 87].
5 Prevention and Management of Allergic Reactions to COVID-19 Vaccines: Recommendations from Scientific Societies and Health Authorities
Several international public health agencies and allergy-related organizations worldwide issued guidance about precautions and contraindications to COVID-19 vaccination, with the ultimate goal of preventing the occurrence of allergic reactions and reducing related concerns and vaccine hesitancy [88].
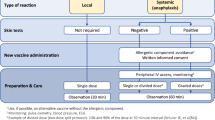
To prevent the onset of allergic reactions following COVID-19 vaccines, there is general agreement about pre-vaccination evaluation by an allergist-immunologist for the risk stratification of an allergic patients (as shown in Table 4) and decide whether vaccination is advisable in high-risk patients. However, limited data are available on how the risk assessment should be performed by the specialist. Furthermore, the evaluation of individuals with a known or suspected history of PEG allergy and who have not yet received an mRNA vaccine differs from those who have already experienced an allergic reaction to the first dose.
According to the World Allergy Organization [89] and to Banerji et al. [63], individuals who report a prior allergic reaction to a vaccine component (PEG or polysorbate 80) or to the first dose, are considered at high risk of allergic reactions. Patients who report history of immediate allergic reactions (especially anaphylaxis) to multiple drug classes with unidentified trigger (as it may be PEG-allergy given its ubiquity as an excipient) or history of anaphylaxis to a PEGylated parenteral monoclonal antibody or history of idiopathic anaphylaxis or a mast cell disease, are included in the intermediate risk group.
In those cases, it is strongly recommended to refer patients to allergist/immunologist. Various diagnostic algorithms, based on clinical assessment and excipient skin testing with PEG and polysorbate 80, have been proposed [63, 68], even if there is no agreement about if and how to perform it. Some allergy-related organizations and health authorities indicate that skin testing is plausible [6,7,8,9,10,11], while others provide no indication [89,90,91,92]. In general, the investigation of potential PEG-related allergy is complex, and both skin prick testing and intradermal testing have been associated with systemic reactions, including anaphylaxis [63, 68, 93]. As such, skin testing should only be performed by specialists with relevant expertise and in the appropriate setting [94].
To investigate the value of excipient skin testing proposed in different diagnostic algorithms, as mentioned above, Greenhawt et al. performed a systematic review yielding a pooled sensitivity of 58.8% and specificity of 99.5% for PEG-allergy detection [95].
In parallel, Wolfson et al. [96] published the first outcomes of the diagnostic algorithm proposed by Banerji et al. [63], which was applied to 80 selected patients who reported an allergic reaction after the first dose of COVID-19 vaccine and underwent allergy assessment with skin testing to PEG and/or polysorbate 80. Overall, in the group of patients who reported an immediate allergic reaction, 89% received the second dose, which was tolerated by most of the vaccinees (74%), while the remaining quarter of vaccinees experienced mild cutaneous allergic reactions. Among those who reported an immediate allergic reaction, only 4 of 65 had a positive PEG skin test result. Of them, two (50%) took and tolerated the vaccine, one was not tested because of severe allergic reaction (anaphylaxis) after the first dose, and one refused to receive the second dose. On the other hand, among patients who reported a history of an immediate allergic reaction and a negative PEG skin test result, 25% had allergic reaction following the second dose of the vaccine. Most reported only cutaneous symptoms, while four (31%) patients were admitted to the emergency department, two of whom were treated with epinephrine; both vaccinees experienced a severe allergic reaction after the first dose. Thus, excipient skin testing did not add value to the clinical risk assessment in those reporting allergic reactions following the first-dose of mRNA COVID-19 vaccine. It has been helpful in the evaluation of individuals with a history of PEG-related anaphylaxis [68, 93], although its positive predictive value remains unknown. Similarly, Greenhawt et al. [95] in the International Consensus mentioned above, recommend against routinely performing skin or in vitro testing using COVID-19 vaccines or excipients outside the research setting, given that such testing has unknown sensitivity/specificity in predicting COVID-19 vaccine-related severe allergic reactions. With regard to the skin test, it is possible that PEG MW may play a role in triggering allergic reactions [68]. A poor diagnostic value of skin testing was also found by Brockow et al. in a very recent study of 421 patients including a retrospective assessment of a cohort of 10 PEG-allergic patients [66].
It has been hypothesized that these preliminary results can be explained by the fact that, to date, there is no other convincing evidence of an IgE-mediated reaction to PEG or polysorbate 80 as triggers for COVID-19 vaccine-allergic reactions and mechanisms. The specific PEG contained in mRNA vaccines, as a stabilizing portion of the liposome, is different, in terms of molecular weight and co-formulation, from PEG that is commonly used in other drugs and healthcare products [64, 97]. Although an exact threshold of reactivity based on the molecular weight of PEG is unknown, tolerance of PEG with molecular weight lower than 400 has been described in those with documented anaphylaxis to PEG-3350 [64]; instead, it is also still unknown if PEG-2000 can be tolerated in these patients. Concerning skin prick testing with the remaining drop of COVID-19 vaccine, preliminary data are still inconclusive.
Cumulative data show that the majority of patients reporting allergic reaction to a COVID-19 vaccine might safely receive the second dose, irrespective of skin test results, and skin tested negative patients might experience an allergic reaction. On the contrary, COVID-19 vaccination in patients who experienced severe allergic reaction to an excipient (PEG or Polisorbate 80) contained in those vaccines has to be contraindicated. The booster dose should be avoided in those who experience a severe allergic reaction following the first COVID-19 vaccine dose, irrespective of negative skin testing results. In the latter case, it has been proposed that vaccines might be administered through graded dosing in a well-equipped setting [95], but more evidence is needed to draw firm conclusions.
Finally, it is commonly agreed that the COVID-19 vaccine or excipient is not contraindicated in patients with a history of severe allergic reactions that are not related to COVID-19 vaccine or excipient, such as in food, insect venom, inhalants and PEG-free drug allergy, and insect venom, as well as in patients with a family history of allergies (Table 4).
Premedication with H1-antihistamine or systemic corticosteroid before receiving COVID-19 vaccine is not recommended as there is no evidence about anaphylaxis prevention; in addition, corticosteroid premedication may lower the immune response following COVID-19 vaccination [95], while antihistamines may mask initial symptoms of a severe allergic reaction [89].
Position papers are important to guide the clinician on ways to handle COVID-19 vaccinations in patients at high-risk for allergic reactions. Ongoing real-life studies on patients seeking allergy workup before COVID-19 vaccine will likely update currently available guidelines [66]. Further research on excipient role, skin testing predictive values and pathogenetic mechanism is particularly needed.
Due to current knowledge gaps regarding COVID-19 allergic reactions, individual benefit/risk ratio evaluated by allergist/immunologist is of utmost importance in the management of patients with allergic reactions and who are to receive or have already received COVID-19 vaccine.
In summary, to date: (i) the decision-making process on administration of subsequent doses of COVID-19 vaccine should be based on the combination of skin test results, allergy clinical history, and the risk/benefit ratio for every single patient; (ii) excipient skin testing alone does not add value to the clinical risk assessment; (iii) the majority of patients reporting an allergic reaction to a COVID-19 vaccine might receive a subsequent dose safely; (iv) patients whose skin tested negative might experience allergic reactions; (v) patients who experienced a severe allergic reaction (anaphylaxis) after COVID-19 vaccination should avoid a subsequent dose of the same vaccine, irrespective of negative skin testing results; in these cases, vaccine administration through graded dosing in a well-equipped setting might be evaluated; vi) severe allergic reaction to an unrelated vaccine, unrelated injectable medication, or due to a food or inhalant, do not contraindicate to the COVID-19 vaccination.
5.1 COVID-19 Vaccine in Patients with Mastocytosis
Mastocytosis is characterized by the proliferation and accumulation of mast cells in the bone marrow and tissues that is accompanied by symptoms of mediator release, such as itching, flushing or more serious symptoms such as gastrointestinal or osteoarticular symptoms or anaphylaxis [98]. Mastocytosis is considered to be a risk factor for the frequency and severity of possible allergic reactions; in fact, patients with suspected mastocytosis often have a history of suspected allergic reactions [99]. The triggers that can cause mast cell degranulation are mainly hymenoptera bites (responsible for about 19%–60% of anaphylaxis in patients with mastocytosis) followed by drugs (5–9%), and food (3–16%) [99,100,101,102]. Patients with mastocytosis may also experience frequent idiopathic anaphylaxis or in the presence of cofactors such as alcohol intake, exercise and sudden changes in temperature [101]. For these reasons, patients are provided with self-injecting adrenaline. For the management of symptoms caused by the release of mast cell mediators, patients can be treated with symptomatic therapy such as antihistamines, antileukotrienes, chromones and, in less frequent cases, also with omalizumab [103]. Obviously, with the start of the COVID-19 vaccination campaign and the first reports of vaccine allergic/anaphylactic reactions, the question arose of how to deal with vaccination for this category of patients [104].
The data already in our possession did not highlight particular problems linked to particularly serious reactions or in any case the presence of risk factors for allergic reactions in patients with mastocytosis with regard to vaccinations in general [2, 105, 106]. For this reason, the European Competence Network on Mastocytosis (ECNM) and the American Initiative in Mast Cell Diseases (AIM) have recently issued an expert opinion on the risk and management of COVID-19 vaccination in patients affected by mastocytosis. Although large and controlled studies are not available, there is consensus in the scientific community that contraindication of the COVID-19 vaccines in adults affected by mastocytosis alone is not necessary [2]. However, as these patients have a greater risk of mast cell degranulation, it is obviously necessary to put in place a series of safety procedures, an adequate period of observation, and possibly have them take anti-mediator therapy as prophylaxis, adapting all this to the individual patient [107].
As these drugs do not exert an immunosuppressive effect, the efficacy of the vaccination is not expected to be reduced, even when those drugs are used for several years. As such, anti–mediator-based treatment should not be discontinued at the time of vaccination.
6 Conclusions
The rate of serious allergic reactions after the administration of all COVID-19 vaccines remains very low, but it appears to be slightly higher compared to traditional vaccines. In general, among COVID19 vaccinees, the risk of developing these reactions appears to be highest in the immediate period following the vaccination and it is higher in women and in those with a history of allergy. As compared to mRNA vaccines, there is little current real-world information on anaphylaxis following COVID-19 viral vector vaccines. Nevertheless, no substantial differences between mRNA and viral vector COVID19 vaccines have been documented. Despite the hypothesis that excipients like polyethylene glycol and polysorbate 80, used in COVID-19 vaccines, could be potential triggers for the onset of allergic reactions, the exact underlying mechanism of mRNA COVID-19 allergic reactions has not been fully elucidated. A risk stratification assessment with the objective of identifying individuals at higher risk of developing serious allergic reactions should always be considered and carried out prior the administration of a vaccine against COVID-19. Protocols for the safe vaccination of individuals at increased risk of serious allergic reactions, such as patients with mastocytosis, should be followed. Given the importance of controlling the pandemic and at the same time ensuring the safety of all subjects receiving COVID-19 vaccines, more evidence is urgently needed, particularly with regard to the safety of vaccine administration in people identified as being at higher risk of developing serious allergic reactions.
References
European Medicines Agency. COVID-19 vaccines: authorised [Internet]. 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section. Accessed 14 Dec 2021.
Nilsson L, Brockow K, Alm J, Cardona V, Caubet J-C, Gomes E, et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28:628–40. https://doi.org/10.1111/pai.12762.
McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–78. https://doi.org/10.1016/j.jaci.2015.07.048.
European Medicines Agency. How the safety of the new COVID-19 vaccines will be monitored [Internet]. 2021. https://www.ema.europa.eu/en/documents/presentation/presentation-how-safety-new-covid-19-vaccines-will-be-monitored-sstraus-prac_en.pdf. Accessed 14 Dec 2021.
Centers for Disease Control and Prevention. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020 [Internet]. 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm?s_cid=mm7002e1_w. Accessed 14 Dec 2021.
World Health Organizationn (WHO). Immunization stress-related response: a manual for program managers and health professionals to prevent, identify and respond to stress-related responses following immunization [Internet]. 2019. https://www.who.int/publications/i/item/9789241515948. Accessed 14 Dec 2021.
Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–2. https://doi.org/10.1001/jama.2021.1967.
Rodriguez-Nava G, Egoryan G, Trelles-Garcia DP, Yanez-Bello MA, Murguia-Fuentes R. Disproportionality analysis of anaphylactic reactions after vaccination with messenger RNA coronavirus disease 2019 vaccines in the United States. Ann Allergy Asthma Immunol. 2021;127(1):139–40. https://doi.org/10.1016/j.anai.2021.04.004.
Evans SJW, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483–6. https://doi.org/10.1002/pds.677.
Sa S, Lee CW, Shim SR, Yoo H, Choi J, Kim JH, et al. The Safety of mRNA-1273, BNT162b2 and JNJ-78436735 COVID-19 vaccines: safety monitoring for adverse events using real-world data. Vaccines (Basel). 2022;10(2):320. https://doi.org/10.3390/vaccines10020320.
Rosenblum HG, Gee J, Liu R, Marquez PL, Zhang B, Strid P, et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect Dis. 2022;S1473–3099(22):00054–8. https://doi.org/10.1016/S1473-3099(22)00054-8.
Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–9. https://doi.org/10.1001/jama.2021.15072.
Brighton collaboration. BC Case Definition: Anaphylaxis (Chinese translation) [Internet]. 2020. https://brightoncollaboration.us/bc-case-definition-anaphylaxis-chinese-translation/. Accessed 14 Dec 2021.
Hwang I, Park K, Kim TE, Kwon Y, Lee Y-K. COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021. Osong Public Health Res Perspect. 2021;12(6):396–402. https://doi.org/10.24171/j.phrp.2021.0310.
Ontario Agency for Health Protection and Promotion (Public Health Ontario). Reports of events managed as anaphylaxis following COVID-19 vaccines in Ontario: December 13, 2020 to March 6, 2021. 2021. https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-anaphylaxis-epi-summary.pdf?la=en. Accessed 14 Dec 2021.
Iguchi T, Umeda H, Kojima M, Kanno Y, Tanaka Y, Kinoshita N, et al. Cumulative adverse event reporting of anaphylaxis after mRNA COVID-19 vaccine (Pfizer-BioNTech) injections in Japan: the first-month report. Drug Saf. 2021;44(11):1209–14. https://doi.org/10.1007/s40264-021-01104-9.
Shavit R, Maoz-Segal R, Iancovici-Kidon M, Offengenden I, Haj Yahia S, Machnes Maayan D, et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4(8): e2122255. https://doi.org/10.1001/jamanetworkopen.2021.22255.
Blumenthal KG, Robinson LB, Camargo CA, Shenoy ES, Banerji A, Landman AB, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–5. https://doi.org/10.1001/jama.2021.3976.
Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–7. https://doi.org/10.1016/j.jaci.2005.12.1303.
Arroliga ME, Dhanani K, Arroliga AC, Huddleston PS, Trahan J, Aguilar T, et al. Allergic reactions and adverse events associated with administration of mRNA-based vaccines. A health-care system experience. Allergy Asthma Proc. 2021;42(5):395–9. https://doi.org/10.2500/aap.2021.42.210069.
Bae S, Lee YW, Lim SY, Lee J-H, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36(17): e115. https://doi.org/10.3346/jkms.2021.36.e115.
Alfaleh A, Alkattan A, Radwan N, Elzohri M, Alzaher A, Ibrahim M, et al. Adverse drug reactions from two COVID-19 vaccines reported in Saudi Arabia. Drugs Ther Perspect [Internet]. 2022;38:84–92. https://doi.org/10.1007/s40267-022-00893-y.
World Health Organizationn (WHO). WHO Coronavirus (COVID-19) Dashboard [Internet]. 2021. https://covid19.who.int/. Accessed 14 Dec 2021.
Uppsala Medical Centre. Your window to a world of global safety insights [Internet]. https://who-umc.org/pv-products/vigilyze/. Accessed 14 Dec 2021.
Meddra.org. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 24.0 [Internet]. 2021. http://alt.meddra.org/files_acrobat/SMQ_intguide_24_0_English.pdf. Accessed 14 Dec 2021.
European Medicines Agency. Comirnaty COVID-19 vaccine: EMA recommends approval for children aged 5 to 11 [Internet]. 2021. https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11. Accessed 14 Dec 2021.
EudraVigilance. European database of suspected adverse drug reaction reports [Internet]. https://www.adrreports.eu/. Accessed 14 Dec 2021.
Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. Longo DL, editor. N Engl J Med. 2021;384(7):643–9. https://doi.org/10.1056/NEJMra2035343.
Banerji A, Wolfson AR, Wickner PG, Cogan AS, McMahon AE, Saff R, et al. COVID-19 vaccination in patients with reported allergic reactions: updated evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(6):2135–8. https://doi.org/10.1016/j.jaip.2021.03.053.
Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–1. https://doi.org/10.1001/jama.2021.0600.
Thong BY-H, Tan T-C. Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol. 2011;71(5):684–700. https://doi.org/10.1111/j.1365-2125.2010.03774.x.
Eaddy Norton A, Broyles AD. Drug allergy in children and adults. Ann Allergy Asthma Immunol. 2019;122(2):148–55. https://doi.org/10.1016/j.anai.2018.11.014.
Trigunaite A, Dimo J, Jørgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294(2):87–94. https://doi.org/10.1016/j.cellimm.2015.02.004.
Fan Z, Che H, Yang S, Chen C. Estrogen and estrogen receptor signaling promotes allergic immune responses: effects on immune cells, cytokines, and inflammatory factors involved in allergy. Allergol Immunopathol (Madr). 2019;47(5):506–12. https://doi.org/10.1016/j.aller.2019.03.001.
Vasiadi M, Kempuraj D, Boucher W, Kalogeromitros D, Theoharides TC. Progesterone inhibits mast cell secretion. Int J Immunopathol Pharmacol. 2006;19(4):787–94. https://doi.org/10.1177/039463200601900408.
Hox V, Desai A, Bandara G, Gilfillan AM, Metcalfe DD, Olivera A. Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric oxide synthase expression and nitric oxide production. J Allergy Clin Immunol. 2015;135(3):729-36.e5. https://doi.org/10.1016/j.jaci.2014.11.003.
Risma KA, Edwards KM, Hummell DS, Little FF, Norton AE, Stallings A, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147(6):2075-2082.e2. https://doi.org/10.1016/j.jaci.2021.04.002.
Alysandratos K, Asadi S, Angelidou A, Zhang B, Sismanopoulos N, Yang H, et al. Neurotensin and CRH interactions augment human mast cell activation. Doherty TM, editor. PLoS ONE. 2012;7(11):e48934. https://doi.org/10.1371/journal.pone.0048934.
Theoharides TC. The impact of psychological stress on mast cells. Ann Allergy, Asthma Immunol. 2020;125(4):388–92. https://doi.org/10.1016/j.anai.2020.07.007.
Jimenez-Rodriguez T, Garcia-Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. 2018;20(11):121–42. https://doi.org/10.2147/JAA.S159411.
Tanno LK, Gonzalez-Estrada A, Olivieri B, Caminati M. Asthma and anaphylaxis. Curr Opin Allergy Clin Immunol. 2019;19(5):447–55. https://doi.org/10.1097/ACI.0000000000000566.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73. https://doi.org/10.1183/09031936.00202013.
Caminati M, Guarnieri G, Senna G. Who is really at risk for anaphylaxis due to COVID-19 vaccine? Vaccines. 2021;9(1):38. https://doi.org/10.3390/vaccines9010038.
Rojas-Pérez-Ezquerra P, Crespo Quirós J, Tornero Molina P, Baeza Ochoa de Ocáriz M, Zubeldia Ortuño J. Safety of new mRNA vaccines against COVID-19 in severely allergic patients. J Investig Allergol Clin Immunol. 2021;31(2):180–1. https://doi.org/10.18176/jiaci.0683.
Fijen LM, Levi M, Cohn DM. COVID-19 vaccination and the risk of swellings in patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(11):4156–8. https://doi.org/10.1016/j.jaip.2021.08.039.
Kounis NG, Koniari I, de Gregorio C, Velissaris D, Petalas K, Brinia A, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines. 2021;9(3):221. https://doi.org/10.3390/vaccines9030221.
Stone CA, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85(12):2694–706. https://doi.org/10.1111/bcp.14112.
Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–22. https://doi.org/10.1111/cea.12760.
Gell PGH, Coombs RRA. Clinical aspects of immunology. Oxford: Blackwell; 1963.
Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014;61(2):163–73. https://doi.org/10.1016/j.molimm.2014.06.038.
European Medicines Agency. Reflection paper on the data requirements for intravenous liposomal products developed with reference to an innovator liposomal product [Internet]. 2013. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-data-requirements-intravenous-liposomal-products-developed-reference-innovator_en.pdf. Accessed 14 Dec 2021
Radice A, Fassio F, Meucci E, Iorno MCL, Macchia D. Potential culprits for immediate hypersensitivity reactions to BNT162b2 mRNA COVID-19 vaccine: not just PEG. Eur Ann Allergy Clin Immunol. 2021;53(5):240–2. https://doi.org/10.23822/EurAnnACI.1764-1489.214.
Ebo DG, Clarke RC, Mertes P-M, Platt PR, Sabato V, Sadleir PHM. Molecular mechanisms and pathophysiology of perioperative hypersensitivity and anaphylaxis: a narrative review. Br J Anaesth. 2019;123(1):e38–49. https://doi.org/10.1016/j.bja.2019.01.031.
Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016;137(6):1674–80. https://doi.org/10.1016/j.jaci.2016.02.015.
Klimek L, Novak N, Hamelmann E, Werfel T, Wagenmann M, Taube C, et al. Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA. Allergo J Int. 2021;30(2):51–5. https://doi.org/10.1007/s40629-020-00160-4.
Ring J, Beyer K, Biedermann T, Bircher A, Fischer M, Fuchs T, et al. Guideline (S2k) on acute therapy and management of anaphylaxis: 2021 update. Allergo J Int. 2021;30(1):1–25. https://doi.org/10.1007/s40629-020-00158-y.
Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140(2):335–48. https://doi.org/10.1016/j.jaci.2017.06.003.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577.
Nopp A, Johansson SGO, Lundberg M, Öman H. Simultaneous exposure of several allergens has an additive effect on multisensitized basophils. Allergy. 2006;61(11):1366–8. https://doi.org/10.1111/j.1398-9995.2006.01211.x.
Wenande E, Kroigaard M, Mosbech H, Garvey LH. Polyethylene glycols (PEG) and related structures. A A Case Rep. 2015;4(5):61–4. https://doi.org/10.1213/XAA.0000000000000126.
Yamasuji Y, Higashi Y, Sakanoue M, Katsue H, Kawai K, Arai N, et al. A case of anaphylaxis caused by polyethylene glycol analogues. Contact Dermatitis. 2013;69(3):183–5. https://doi.org/10.1111/cod.12084.
Sellaturay P, Gurugama P, Harper V, Dymond T, Ewan P, Nasser S. The Polysorbate containing AstraZeneca COVID-19 vaccine is tolerated by polyethylene glycol (PEG) allergic patients. Clin Exp Allergy. 2022;52(1):12–7. https://doi.org/10.1111/cea.14064.
Banerji A, Wickner PG, Saff R, Stone CA, Robinson LB, Long AA, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–37. https://doi.org/10.1016/j.jaip.2020.12.047.
Stone CA, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533-1540.e8. https://doi.org/10.1016/j.jaip.2018.12.003.
Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51(6):861–3. https://doi.org/10.1111/cea.13874.
Brockow K, Mathes S, Fischer J, Volc S, Darsow U, Eberlein B, et al. Experience with polyethylene glycol allergy-guided risk management for COVID-19 vaccine anaphylaxis. Allergy. 2021. https://doi.org/10.1111/all.15183.
Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126(3):e106–8. https://doi.org/10.1016/j.bja.2020.12.020.
Sellaturay P, Nasser S, Ewan P. Polyethylene glycol-induced systemic allergic reactions (Anaphylaxis). J Allergy Clin Immunol Pract. 2021;9(2):670–5. https://doi.org/10.1016/j.jaip.2020.09.029.
Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, et al. Antibody against poly (ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110(1):103–11. https://doi.org/10.1002/cncr.22739.
Yang Q, Jacobs TM, McCallen JD, Moore DT, Huckaby JT, Edelstein JN, et al. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal Chem. 2016;88(23):11804–12. https://doi.org/10.1021/acs.analchem.6b03437.
Hsieh Y-C, Wang H-E, Lin W-W, Roffler SR, Cheng T-C, Su Y-C, et al. Pre-existing anti-polyethylene glycol antibody reduces the therapeutic efficacy and pharmacokinetics of PEGylated liposomes. Theranostics. 2018;8(11):3164–75. https://doi.org/10.7150/thno.22164.
Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9(11):1319–23. https://doi.org/10.1517/17425247.2012.720969.
Elsadek NE, Lila ASA, Emam SE, Shimizu T, Takata H, Ando H, et al. Pegfilgrastim (PEG-G-CSF) induces anti-PEG IgM in a dose dependent manner and causes the accelerated blood clearance (ABC) phenomenon upon repeated administration in mice. Eur J Pharm Biopharm. 2020;152:56–62. https://doi.org/10.1016/j.ejpb.2020.04.026.
Ishida T, Kiwada H. Anti-polyethyleneglycol antibody response to PEGylated substances. Biol Pharm Bull. 2013;36(6):889–91. https://doi.org/10.1248/bpb.b13-00107.
Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FHCB. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292:1071–9.
Shimizu T, Ichihara M, Yoshioka Y, Ishida T, Nakagawa S, Kiwada H. Intravenous Administration of Polyethylene Glycol-Coated (PEGylated) Proteins and PEGylated Adenovirus Elicits an Anti-PEG Immunoglobulin M Response. Biol Pharm Bull. 2012;35(8):1336–42. https://doi.org/10.1248/bpb.b12-00276.
Eckardt K-U. The safety and efficacy of peginesatide in patients with CKD. Nat Rev Nephrol. 2013;9(4):192–3. https://doi.org/10.1038/nrneph.2013.42.
Mikhail A. Profile of peginesatide and its potential for the treatment of anemia in adults with chronic kidney disease who are on dialysis. J Blood Med. 2012;3:25–31. https://doi.org/10.2147/JBM.S23270.
Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20(1):710–24. https://doi.org/10.1080/14686996.2019.1627174.
Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):655–77. https://doi.org/10.1002/wnan.1339.
Wenande EC, Skov PS, Mosbech H, Poulsen LK, Garvey LH. Inhibition of polyethylene glycol–induced histamine release by monomeric ethylene and diethylene glycol: a case of probable polyethylene glycol allergy. J Allergy Clin Immunol. 2013;131(5):1425–7. https://doi.org/10.1016/j.jaci.2012.09.037.
Hamad I, Hunter AC, Szebeni J, Moghimi SM. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol Immunol. 2008;46(2):225–32. https://doi.org/10.1016/j.molimm.2008.08.276.
Szebeni J, Muggia F, Gabizon A, Barenholz Y. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv Drug Deliv Rev. 2011;63(12):1020–30. https://doi.org/10.1016/j.addr.2011.06.017.
Ishida T, Kashima S, Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J Control Release. 2008;126(2):162–5. https://doi.org/10.1016/j.jconrel.2007.11.009.
Warren CM, Snow TT, Lee AS, Shah MM, Heider A, Blomkalns A, et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US Regional Health System. JAMA Netw Open. 2021;4(9): e2125524. https://doi.org/10.1001/jamanetworkopen.2021.25524.
Troelnikov A, Perkins G, Yuson C, Ahamdie A, Balouch S, Hurtado PR, et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J Allergy Clin Immunol. 2021;148(1):91–5. https://doi.org/10.1016/j.jaci.2021.04.032.
Inglut CT, Sorrin AJ, Kuruppu T, Vig S, Cicalo J, Ahmad H, et al. Immunological and toxicological considerations for the design of liposomes. Nanomaterials. 2020;10(2):190. https://doi.org/10.3390/nano10020190.
Robinson LB, Landman AB, Shenoy ES, Hashimoto D, Fu X, Camargo CA, et al. Allergic symptoms after mRNA COVID-19 vaccination and risk of incomplete vaccination. J Allergy Clin Immunol Pract. 2021;9(8):3200-3202.e1. https://doi.org/10.1016/j.jaip.2021.05.031.
Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, El-Gamal Y, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14(2): 100517. https://doi.org/10.1016/j.waojou.2021.100517.
Sokolowska M, Eiwegger T, Ollert M, Torres MJ, Barber D, Del Giacco S, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy. 2021;76(6):1629–39. https://doi.org/10.1111/all.14739.
Government of Canada. Recommendations on the use of COVID-19 vaccines [Internet]. 2021. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/table-updates.html. Accessed 14 Dec 2021
Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States - Appendix B: Triage of people with a history of allergies or allergic reactions [Internet]. 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#Appendix-B. Accessed 14 Dec 2021
Wylon K, Dölle S, Worm M. Polyethylene glycol as a cause of anaphylaxis. Allergy, Asthma Clin Immunol. 2016;13(12):67. https://doi.org/10.1186/s13223-016-0172-7.
gov.uk. COVID-19 - SARS-CoV-2 [Internet]. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1040677/Greenbook-chapter-14a-14Dec21.pdf. Accessed 14 Dec 2021
Greenhawt M, Abrams EM, Shaker M, Chu DK, Khan D, Akin C, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546–67. https://doi.org/10.1016/j.jaip.2021.06.006.
Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308-3320.e3. https://doi.org/10.1016/j.jaip.2021.06.010.
Krantz MS, Liu Y, Phillips EJ, Stone CA. Anaphylaxis to PEGylated liposomal echocardiogram contrast in a patient with IgE-mediated macrogol allergy. J Allergy Clin Immunol Pract. 2020;8(4):1416-1419.e3. https://doi.org/10.1016/j.jaip.2019.12.041.
Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: Consensus Statements on Diagnostics, Treatment Recommendations and Response Criteria. Eur J Clin Invest. 2007;37(6):435–53. https://doi.org/10.1111/j.1365-2362.2007.01807.x.
Pieri L, Bonadonna P, Elena C, Papayannidis C, Grifoni FI, Rondoni M, et al. Clinical presentation and management practice of systemic mastocytosis. A survey on 460 Italian patients. Am J Hematol. 2016;91(7):692–9. https://doi.org/10.1002/ajh.24382.
González Olano D, de la Hoz Caballer B, Núñez López R, Sánchez Muñoz L, Cuevas Agustín M, Diéguez MC, et al. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA). Clin Exp Allergy. 2007;37(10):1547–55. https://doi.org/10.1111/j.1365-2222.2007.02804.x.
Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63(2):226–32. https://doi.org/10.1111/j.1398-9995.2007.01569.x.
Gülen T, Hägglund H, Dahlén B, Nilsson G. High prevalence of anaphylaxis in patients with systemic mastocytosis—a single-centre experience. Clin Exp Allergy. 2014;44(1):121–9. https://doi.org/10.1111/cea.12225.
Broesby-Olsen S, Vestergaard H, Mortz CG, Jensen B, Havelund T, Hermann AP, et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: efficacy and safety observations. Allergy. 2018;73(1):230–8. https://doi.org/10.1111/all.13237.
Food and Drug Administration. Moderna COVID-19 Vaccine [Internet]. 2020. https://www.fda.gov/media/144434/download. Accessed 14 Dec 2021.
Carter MC, Metcalfe DD, Matito A, Escribano L, Butterfield JH, Schwartz LB, et al. Adverse reactions to drugs and biologics in patients with clonal mast cell disorders: a Work Group Report of the Mast Cells Disorder Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2019;143(3):880–93. https://doi.org/10.1016/j.jaci.2018.10.063.
Zanoni G, Zanotti R, Schena D, Sabbadini C, Opri R, Bonadonna P. Vaccination management in children and adults with mastocytosis. Clin Exp Allergy. 2017;47(4):593–6. https://doi.org/10.1111/cea.12882.
Bonadonna P, Brockow K, Niedoszytko M, Elberink HO, Akin C, Nedoszytko B, et al. COVID-19 Vaccination in mastocytosis: recommendations of the European Competence Network on Mastocytosis (ECNM) and American Initiative in Mast Cell Diseases (AIM). J Allergy Clin Immunol Pract. 2021;9(6):2139–44. https://doi.org/10.1016/j.jaip.2021.03.041.
Dézsi L, Szénási G, Urbanics R, Rosivall L, Szebeni J. Cardiopulmonary and hemodynamic changes in complement activation-related pseudoallergy. Health (Irvine Calif). 2013;05:1032–8. https://doi.org/10.4236/health.2013.56138.
European Medicines Agency. Treatments and vaccines for COVID-19 [Internet]. 2021. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines-covid-19. Accessed 14 Dec 2021.
American Academy of Allergy Asthma & Immunology. AAAAI COVID-19 Response Task Force Guidance on Administration of COVID-19 Vaccines Related to Concerns of Allergic Reactions [Internet]. 2021. https://education.aaaai.org/resources-for-a-i-clinicians/reactionguidance_COVID-19. Accessed 14 Dec 2021.
American College of Allergy Asthma & Immunology. ACAAI Updates to Guidance on Risk of Allergic Reactions to COVID-19 Vaccines [Internet]. 2021. https://acaai.org/news/acaai-updates-to-guidance-on-risk-of-allergic-reactions-to-covid-19-vaccines/. Accessed 14 Dec 2021.
Australasian Society of Clinical Immunology and Allergy. Guide: Allergy and COVID-19 Vaccination [Internet]. 2021. https://www.allergy.org.au/hp/papers/guide-allergy-and-covid-19-vaccination. Accessed 14 Dec 2021.
Canadian Society of Allergy and Clinical Immunology. COVID-19 Vaccine Testing & Administration Guidance for Allergists/Immunologists from the CSACI [Internet]. 2021. https://csaci.ca/wp-content/uploads/2021/01/COVID-19-VaccineTesting-AdministrationGuidance-JAN5.pdf. Accessed 14 Dec 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Conflicts of interest
Gianluca Trifirò has served in the last three years on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, AbbVie, Servier, Mylan, Gilead, Amgen; he was the scientific director of a Master program on pharmacovigilance, pharmacoepidemiology and real-world evidence which has received non-conditional grant from various pharmaceutical companies; he coordinated a pharmacoepidemiology team at the University of Messina until Oct 2020, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daichii Sankyo, PTC Pharmaceuticals). He is also scientific coordinator of the academic spin-off "INSPIRE srl" which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). All the above-mentioned activities are not related to the topic of the manuscript. Carmen Ferrajolo declares that she is a member and vice-chief of academic Spin-off "INSPIRE SRL– INnovative Solutions for medical Prediction and big data Integration in REal world setting SRL which has received funding for conducting observational studies from various pharmaceutical companies. All the above-mentioned activities are not related to the topic of the manuscript. The other authors have no conflicts of interest to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Author contributions
GT, NL, AG contributed to the conception and the design of the article. NL and AG performed the literature search for the allergic reaction data from observational studies. PMC, CF, UM, GP performed the search through the spontaneous reporting databases. GS and FF performed the literature search for the identification of risk factors from the real-world setting data. MC and LG described the role of PEGylation. GZ and AA reviewed the literature and summarized the recommendation from the scientific societies and health authorities. PB and EO searched and reviewed the literature for COVID-19 in high-risk groups. All authors reviewed the results and approved the final version of the manuscript. GT approved this version to be published.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Luxi, N., Giovanazzi, A., Arcolaci, A. et al. Allergic Reactions to COVID-19 Vaccines: Risk Factors, Frequency, Mechanisms and Management. BioDrugs 36, 443–458 (2022). https://doi.org/10.1007/s40259-022-00536-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00536-8