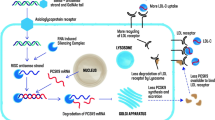
Abstract
Hypercholesterolemia is a leading cause of cardiovascular disease and mortality in men and women throughout the USA and abroad. The development of statins (HMG-CoA reductase inhibitors) to lower plasma atherogenic cholesterol levels and improve cardiovascular outcomes represents one of the greatest contributions to clinical science in the twentieth century, although residual risk remains even among statin-treated patients. Our understanding of lipid metabolism took a giant leap forward in 2003 with the discovery of proprotein convertase subtilsin/kexin type 9 (PCSK9), a low abundance circulating protein with an outsized effect on the regulation of plasma cholesterol levels. Evolocumab and alirocumab represent the first two US Food and Drug Administration-approved fully human monoclonal antibodies that target PCSK9, which not only lower low-density lipoprotein (LDL) cholesterol to unprecedented levels, but also further improve important cardiovascular outcomes. Small interfering RNA (siRNA) molecules now represent the next generation of drugs designed to antagonize PCSK9. Inclisiran is a siRNA specific for PCSK9 that prevents translation of PCSK9 messenger RNA, leading to decreased concentrations of the protein and lower concentrations of LDL cholesterol. The ORION clinical development program includes several completed and ongoing clinical trials designed to evaluate the safety and efficacy of inclisiran and test its ability to improve hard cardiovascular outcomes. This review discusses the mechanisms of action of inclisiran, examines the current evidence, and provides a comparison of similarities and differences relative to the PCSK9 inhibitors.

Similar content being viewed by others
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–528.
GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–724.
Ford ES, Capewell S. Trends in total and low-density lipoprotein cholesterol among U.S. adults: contributions of changes in dietary fat intake and use of cholesterol-lowering medications. PLoS One. 2013;8(5):e65228.
Maddox TM, Chan PS, Spertus JA, Tang F, Jones P, Ho PM, et al. Variations in coronary artery disease secondary prevention prescriptions among outpatient cardiology practices: insights from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2014;63(6):539–46.
Hirsh BJ, Smilowitz NR, Rosenson RS, Fuster V, Sperling LS. Utilization of and adherence to guideline-recommended lipid-lowering therapy after acute coronary syndrome: opportunities for improvement. J Am Coll Cardiol. 2015;66(2):184–92.
Laboy SM, Pulley MT. Statin-associated muscle symptoms: does the benefit outweigh the risk factor? Muscle Nerve. 2019;59(5):525–7.
Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–6.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72.
Shapiro MD, Fazio S. From lipids to inflammation: new approaches to reducing atherosclerotic risk. Circ Res. 2016;118(4):732–49.
Chaudhary R, Garg J, Shah N, Sumner A. PCSK9 inhibitors: a new era of lipid lowering therapy. World J Cardiol. 2017;9(2):76–91.
Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–84.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22.
Ference BA, Graham I, Tokgozoglu L, Catapano AL. Reprint of: Impact of lipids on cardiovascular health: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(23 Pt B):2980–95.
Timms KM, Wagner S, Samuels ME, Forbey K, Goldfine H, Jammulapati S, et al. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum Genet. 2004;114(4):349–53.
Shioji K, Mannami T, Kokubo Y, Inamoto N, Takagi S, Goto Y, et al. Genetic variants in PCSK9 affect the cholesterol level in Japanese. J Hum Genet. 2004;49(2):109–14.
Alves AC, Etxebarria A, Medeiros AM, Benito-Vicente A, Thedrez A, Passard M, et al. Characterization of the first PCSK9 gain of function homozygote. J Am Coll Cardiol. 2015;66(19):2152–4.
Iacocca MA, Wang J, Sarkar S, Dron JS, Lagace T, McIntyre AD, et al. Whole-gene duplication of PCSK9 as a novel genetic mechanism for severe familial hypercholesterolemia. Can J Cardiol. 2018;34(10):1316–24.
Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37(2):161–5.
Fasano T, Cefalu AB, Di Leo E, Noto D, Pollaccia D, Bocchi L, et al. A novel loss of function mutation of PCSK9 gene in white subjects with low-plasma low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 2007;27(3):677–81.
Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79(3):514–23.
Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 2007;193(2):445–8.
Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376(16):1527–39.
Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376(16):1517–26.
Kosmas CE, Munoz Estrella A, Sourlas A, Silverio D, Hilario E, Montan PD, et al. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases. 2018;6(3):63.
Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–55.
Bernards R. Exploring the uses of RNAi–gene knockdown and the Nobel Prize. N Engl J Med. 2006;355(23):2391–3.
Kosmas CE, DeJesus E, Morcelo R, Garcia F, Montan PD, Guzman E. Lipid-lowering interventions targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): an emerging chapter in lipid-lowering therapy. Drugs Context. 2017;6:212511.
Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–61.
Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383(9911):60–8.
Fitzgerald K, Kallend D, Simon A. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(18):e38.
Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376(15):1430–40.
Ray KK, Stoekenbroek RM, Kallend D, Nishikido T, Leiter LA, Landmesser U, et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels: one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol. Epub. 2019. https://doi.org/10.1001/jamacardio.2019.3502.
The Medicines Company. ORION clinical development program. Updated 12 March 2019. https://www.themedicinescompany.com/clinical-development/. Accessed 5 Nov 2019.
University of Oxford. A randomized trial assessing the effects of inclisiran on clinical outcomes among people with cardiovascular disease [ClinicalTrials.gov identifier NCT03705234]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03705234. Accessed 5 Nov 2019.
The Medicines Company. A study of inclisiran in participants with homozygous familial hypercholesterolemia (HoFH) [ClinicalTrials.gov identifier NCT03851705]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03851705. Accessed 5 Nov 2019.
The Medicines Company. Trial to evaluate the effect of inclisiran treatment on low density lipoprotein cholesterol (LDL-C) in subjects with heterozygous familial hypercholesterolemia (HeFH) [ClinicalTrials.gov identifier NCT03397121]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03397121. Accessed 5 Nov 2019.
The Medicines Company. Inclisiran for participants with atherosclerotic cardiovascular disease and elevated low-density lipoprotein cholesterol [ClinicalTrials.gov identifier NCT03399370]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03399370. Accessed 5 Nov 2019.
The Medicines Company. Inclisiran for subjects With ACSVD or ACSVD-risk equivalents and elevated low-density lipoprotein cholesterol [ClinicalTrials.gov identifier NCT03400800]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03400800. Accessed 5 Nov 2019.
Ray K. The ORION-11 trial. European Society of Cardiology Congress 2019. 31 Aug–4 Sep 2019; Paris.
The Medicines Company. An extension trial of inclisiran compared to evolocumab in participants with cardiovascular disease and high cholesterol [ClinicalTrials.gov identifier NCT03060577]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03060577. Accessed 5 Nov 2019.
The Medicines Company. Trial to assess the effect of long term dosing of inclisiran in subjects with high CV risk and elevated LDL-C [ClinicalTrials.gov identifier NCT03814187]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03814187. Accessed 5 Nov 2019.
The Medicines Company. A study of ALN-PCSSC in participants with homozygous familial hypercholesterolemia (HoFH) [ClinicalTrials.gov identifier NCT02963311]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT02963311. Accessed 5 Nov 2019.
Raal F, Lepor N, Kallend D, Stoekenbroek R, Wijngaard P, Hovingh GK. Inclisiran durably lowers Ldl-C And Pcsk9 expression in subjects with homozygous familial hypercholesterolaemia: the Orion-2 pilot study. Atherosclerosis. 2019;287:e7.
The Medicines Company. A study of inclisiran in participants with renal impairment compared to participants with normal renal function (ORION-7) [ClinicalTrials.gov identifier NCT03159416]. National Institutes of Health, ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03159416. Accessed 5 Nov 2019.
Kallend D, Collins M, Kastelein J, Landmesser U, Leiter L, Ray K, et al. Efficacy, safety and pharmacokinetics of inclisiran by renal function. Atherosclerosis. 2019;287:e38.
Henne KR, Ason B, Howard M, Wang W, Sun J, Higbee J, et al. Anti-PCSK9 antibody pharmacokinetics and low-density lipoprotein-cholesterol pharmacodynamics in nonhuman primates are antigen affinity-dependent and exhibit limited sensitivity to neonatal Fc receptor-binding enhancement. J Pharmacol Exp Ther. 2015;353(1):119–31.
Shapiro MD, Tavori H, Fazio S. PCSK9: from basic science discoveries to clinical trials. Circ Res. 2018;122(10):1420–38.
Shapiro MD, Miles J, Tavori H, Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Michael Shapiro is supported by NIH K12HD043488.
Conflict of interest
Michael Shapiro reports compensation for advisory activities from Esperion and consulting with Amarin. Charles German has no disclosures.
Rights and permissions
About this article
Cite this article
German, C.A., Shapiro, M.D. Small Interfering RNA Therapeutic Inclisiran: A New Approach to Targeting PCSK9. BioDrugs 34, 1–9 (2020). https://doi.org/10.1007/s40259-019-00399-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-019-00399-6