Abstract
Multicomponent meningococcal serogroup B vaccine (4CMenB; Bexsero®) is a unique vaccine containing four main immunogenic components: three recombinant proteins combined with outer membrane vesicles derived from meningococcal NZ98/254 strain. After three doses of 4CMenB (administered at 2, 3, and 4 months or 2, 4, and 6 months of age) in vaccine-naive infants, the majority of infants had seroprotective human complement serum bactericidal assay (hSBA) antibody titers against the meningococcal serogroup B test strains selected to be specific for the vaccine antigens in randomized, open-label or observer-blind, multicenter, phase IIb or III trials. In extensions to the phase III trial, two doses of 4CMenB administered between 12 and 15 months of age in vaccine-naive infants, and a single booster dose of 4CMenB administered at 12 months of age in vaccine-experienced infants, also elicited robust immunogenic responses. In a phase IIb/III trial, the majority of adolescents (aged 11–17 years) achieved seroprotective hSBA antibody titers against meningococcal serogroup B test strains after two doses of 4CMenB, and a third dose did not appear to add any extra protection. In adults who were potentially at an increased risk of occupational exposure to meningococcal isolates, seroprotection rates were high after one dose of 4CMenB and increased further after two or three doses in a small noncomparative, two-center, phase II trial. The reactogenicity of 4CMenB was generally acceptable in clinical trials. However, the vaccine was associated with more solicited systemic adverse events (particularly fever) in infants when coadministered with routine infant vaccines than when these vaccines were administered alone. In conclusion, 4CMenB effectively elicited immune responses against meningococcal serogroup B test strains selected to be specific for the vaccine antigens in infants, adolescents, and adults.
Similar content being viewed by others
A unique meningococcal serogroup B vaccine containing four main immunogenic components |
Elicits strong immune responses against vaccine components in infants, adolescents, and adults |
May be coadministered with routine infant vaccines without affecting the immunogenicity of either vaccine |
Has generally acceptable tolerability in infants, adolescents, and adults |
In infants, solicited systemic reactions (particularly fever) are more frequent with 4CMenB when coadministered with routine infant vaccines than when these vaccines are administered alone |
1 Introduction
Invasive meningococcal disease (IMD) is a potentially life-threatening Neisseria meningitidis infection which presents most commonly as meningococcal bacteremia and/or, in about half of infected patients, as meningitis [1]. Although the incidence of IMD is rare [2, 3], with overall notification rates of 0.92 per 100,000 population in the EU in 2009 [3], the disease carries with it a high mortality and morbidity rate [4]. In the EU in 2009, the overall case fatality rate was 6–10 % [3]. Moreover, US estimates indicate that 12–19 % of IMD survivors are left with long-term morbidities (e.g. hearing loss, brain damage, renal failure, or limb amputation) [1].
There are 13 different serogroups of N. meningitidis, and serogroups A, B, C, W135, and Y are known to be responsible for >90 % of IMD worldwide, including sporadic and epidemic disease [2, 5]; serogroup X also has epidemic potential [2]. The key strategy for controlling meningococcal disease is primary prevention with effective vaccination programs [6]. Vaccines targeting the serogroup C capsular polysaccharide (monovalent vaccines) [7, 8] or the serogroup A, C, W135, and Y capsular polysaccharides (quadrivalent vaccine) [9] of N. meningitidis are available, and meningococcal serogroup C conjugate vaccines have become part of the routine infant immunization schedule in many countries, including the UK and various other European countries [10].
In the UK during the late 1990s, the incidence of serogroup C disease was increasing because of the introduction and spread of a hyperinvasive, hypervirulent clone [6]. Introduction of meningococcal serogroup C conjugate vaccines into the routine infant immunization schedule (and a catch-up campaign in children and adolescents aged <18 years) in 1999 resulted in a sharp decline in the overall number and proportion of cases of serogroup C disease observed over the ensuing years [6, 11]. In addition to active immunization, herd immunity was shown to play a role in this decline [6, 11, 12]. A similar pattern of events was observed in many other European countries [6].
Most meningococcal disease in Europe is caused by N. meningitidis serogroup B or C, and serogroup B disease accounts for the highest proportion of cases (71 % in the EU in 2009 versus 13 % for serogroup C) [3]. This is due, in part, to the successful reduction in serogroup C disease by effective vaccination programs [11, 13]. Development of effective vaccines against serogroup B disease has been slow because the serogroup B capsular polysaccharide is a poor immunogen and also has the potential to be an autoantigen [6, 13]. Therefore, the focus has been on developing vaccines that target noncapsular meningococcal antigens [6, 13].
Multicomponent meningococcal serogroup B vaccine (4CMenB; Bexsero®) is a unique vaccine containing four main immunogenic components: two recombinant fusion proteins, Neisserial heparin-binding antigen (NHBA; formerly known as genome-derived Neisserial antigen [GNA]-2132) fused with GNA1030, and factor H binding protein (fHbp) fused with GNA2091; recombinant Neisserial adhesin A (NadA); and detergent-treated outer membrane vesicles (OMV) derived from meningococcal NZ98/254 strain (New Zealand outbreak strain), in which PorA is the major immunodominant antigen [13–15]. NHBA, fHbp, and NadA are N. meningitidis surface-exposed proteins that were identified via a novel antigen discovery method known as whole-genome screening (or genome mining) [16, 17].
4CMenB is approved in the EU for active immunization against disease caused by N. meningitidis serogroup B in individuals aged ≥2 months [18]. This article reviews the immunogenicity and reactogenicity of 4CMenB in healthy infants, adolescents, and adults.
Sources:
Medical literature (including published and unpublished data) on ‘multicomponent meningococcal serogroup B vaccine’ was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) [searches last updated 25 February 2013], bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug.
Search terms: ‘multicomponent meningococcal serogroup B vaccine’, ‘meningococcal serogroup B’, ‘Bexsero’, ‘MenACWY-CRM’, ‘4CMENB’, ‘multicomponent meningococcal serogroup B’, ‘meningococcal AND serogroup B’.
Study selection: Studies in infants, adolescents, and adults who received primary or booster vaccination with multicomponent meningococcal serogroup B vaccine. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred.
Keywords: Bexsero®, 4CMenB, multicomponent meningococcal serogroup B vaccine, Neisseria meningitidis serogroup B, immunogenicity, reactogenicity.
2 Immunogenicity
Results of two phase II trials conducted in healthy infants (aged ≤12 months; n = 60,147) demonstrated that three doses of 4CMenB had a greater immunogenicity [19] or broader coverage [20] than a formulation of the vaccine containing all of the same immunogenic components as 4CMenB except the OMV component. Based on these favorable findings, 4CMenB was the formulation evaluated in further trials.
This section focuses on the immunogenicity of 4CMenB vaccine in healthy infants or adolescents, as evaluated in randomized, open-label [21, 22] or observer-blind [23], multicenter, phase IIb [22], IIb/III [23], or III [21] studies, and in healthy adults (laboratory workers, who were potentially at an increased risk of occupational exposure to meningococcal isolates) in a small noncomparative, two-center phase II study [24]. Data from extensions to the phase III trial are also discussed [21, 25, 26]. See Table 1 for key trial design details and the vaccine regimens administered in these studies.
The vaccine components contained in 4CMenB and the other vaccines administered in the trials are shown in Table 2. All vaccines were administered intramuscularly [21–24], except for the measles, mumps, rubella, and varicella vaccine (MMRV), which was administered subcutaneously [21].
In general, immunogenicity was assessed in serum samples collected prior to the first dose of study vaccine and 1 month after the final dose of study vaccine [21–25]; however, two studies also assessed immunogenicity 1 month after each dose of study vaccine [23, 24] and/or immediately prior to the last dose of study vaccine [24]. In the phase III booster study, immunogenicity against MMRV components was assessed in serum samples collected 2 months after MMRV vaccination [21, 26].
Key immunogenicity endpoints included seroprotection rates, geometric mean titers (GMTs) against meningococcal serogroup B test strains (as measured by the human complement serum bactericidal assay [hSBA]) selected to be specific for the individual antigens contained in 4CMenB, and/or the geometric mean ratio (GMR) of hSBA GMTs between post- and pre-vaccination serum samples [21–25]. All studies measured hSBA titers against meningococcal serogroup B test strains specific for three of the antigens contained in 4CMenB: 44/76-SL for fHbp, 5/99 for NadA, and NZ98/254 for PorA P1.4 of OMV [21–25]. In primary and booster phases of the phase III trial in infants, hSBA GMTs against a test strain considered to be specific for the fourth 4CMenB antigen were also measured (i.e. M10713 for NHBA) [21] and, in the catch-up trial, antibodies to NHBA were assessed by ELISA [25]. An hSBA titer of ≥4 was considered to be seroprotective in most studies [21, 23, 24]; however, an hSBA titer of ≥5 was used as the seroprotective cut-off in some studies [21, 22] because, where specified, it was considered to give 95 % confidence for seroprotection [21].
The primary phase of the phase III infant study also investigated the lot-to-lot consistency of three different lots of 4CMenB [21]. Lot-to-lot consistency across the three 4CMenB lots assessed was confirmed when the 95 % confidence interval of the ratio of hSBA GMTs against fHbp, NadA, and PorA for all three pairs of lots (i.e. lot 1:lot 2, lot 1:lot 3, and lot 2:lot 3) was within the predefined limits of 0.5–2.0; the lowest 95 % confidence interval lower limit was 0.74 and the highest 95 % confidence interval upper limit was 1.33.
2.1 In Infants
4CMenB elicited a strong immune response against fHbp, NadA, and PorA in infants in a phase IIb [22] and III [21, 25] study; where assessed, the immune response against NHBA was also strong [21, 25].
2.1.1 Phase III Trial
2.1.1.1 Primary Trial
At baseline, seroprotective titers against fHbp, NadA, and PorA were seen in 3.0 %, 4.3 %, and 1.2 % of infants; in the subgroup (n = 100) of infants tested (post hoc) for response against NHBA, 33 % had seroprotective titers against NHBA at baseline [21]. In the group of infants who only received routine vaccines in this trial (the control group, i.e. group 4), seroprotective titers were seen in 2–3 % of infants at 7 months of age, indicating that seroprotective responses did not evolve during the study period in either treatment arm.
One month after the third dose of 4CMenB, all infants achieved seroprotective hSBA titers against fHbp and NadA, and 84 % achieved seroprotective titers against PorA [21]. The lower limits of the 95 % confidence interval for each of these percentages were all above the prespecified limit of ≥70 %, indicating a sufficient immune response against the three test strains. In the subgroup of infants assessed for response against NHBA, 84 % achieved seroprotective titers 1 month after the third 4CMenB dose [21]. Pre- and post-dose hSBA GMTs and the GMR of hSBA GMTs post- to pre-dose are shown in Table 3.
In addition, the immunogenic response to routine infant vaccines (e.g. the combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus, and Haemophilus influenzae type b [6-in-1] vaccine) was not affected to a clinically relevant extent by concomitant administration with 4CMenB, with responses (definition varied with antigen) reaching accepted thresholds and being similar in infants who received the routine vaccines alone or concomitantly with 4CMenB [21].
2.1.1.2 Extension Studies
Prior to administration of a 4CMenB booster dose, the majority (≥81 %) of 12-month-old infants who had previously received three doses of 4CMenB in the primary trial had seroprotective hSBA titers against fHbp and NadA [21] (Table 4). Seroprotection rates were ≤61 % for the other two test strains. One month following the 4CMenB booster dose, seroprotective titers against the four test strains were seen in 95–100 % of subjects [21] (Table 4). Post-dose hSBA GMTs and the GMR of hSBA GMTs post- to pre-dose are shown in Table 4.
In 12-month-old infants who had previously participated in the primary trial but had not received 4CMenB (i.e. were randomized to the control group), ≤5 % had seroprotective hSBA titers against fHbp, NadA, and PorA prior to the first of two catch-up doses of 4CMenB (data available as a poster) [25] (Table 4). However, 1 month after the second catch-up dose of 4CMenB, seroprotective hSBA titers against the three test strains were seen in ≥96 % of infants [25] (Table 4). Antibodies to NHBA (assessed by ELISA) were increased >279-fold after two doses of 4CMenB [25]. Post-dose hSBA GMTs and the GMR of hSBA post- to pre-dose are shown in Table 4.
Coadministration of 4CMenB with (as opposed to without) MMRV did not appear to alter the immunogenicity of 4CMenB. For example, post-dose seroprotection rates against test strains were generally similar among subjects who did or did not receive concomitant MMRV in the catch-up dose study (Table 4) [25].
In addition, results of the booster study demonstrated that the immunogenic response to the MMRV vaccine was not affected by concomitant administration with 4CMenB (data available as a poster) [26]. Regardless of whether infants received MMRV with or without 4CMenB, responses (definition varied with antigen) to each of the four components of the MMRV vaccine were seen in 96–100 % of subjects [26].
2.1.2 Phase IIb Trial
After three doses of 4CMenB (administered at 2, 4, and 6 months or 2, 3, and 4 months of age with or without the 6-in-1 and 7-valent pneumococcal conjugate [PCV7] vaccines) [i.e. groups 1–3], seroprotection rates against fHbp and NadA were >99 % in all three 4CMenB-receiving groups, and those against PorA were ≥79 % [22]. Seroprotection rates in the control group (those who only received routine vaccines, i.e. group 4) were ≤5.3 % for all three test strains. The lower limit of the 95 % confidence interval for the seroprotection rates was ≥70 % for all three test strains in all 4CMenB-receiving groups; thus, according to prespecified criteria, all vaccine regimens containing 4CMenB were considered to have elicited a sufficient immune response.
4CMenB administered concomitantly with 6-in-1 and PCV7 at 2, 4, and 6 months (i.e. group 1) was noninferior to 4CMenB administered separately to 6-in-1 and PCV7 at 2, 4, and 6 months (i.e. group 2) with regard to seroprotection rates against fHbp and NadA, but not PorA [22]. Noninferiority for response against fHbp and NadA was established because the lower limit of the 95 % confidence interval for the difference in seroprotection rates between the two groups (concomitant minus separate) was greater than −10 % for both of these test strains. For PorA, the between-group difference in seroprotection rates was −7.1 % (95 % CI −11.7 % to −2.6 %) [22].
In general, coadministration of 4CMenB with 6-in-1 and PCV7 did not appear to affect the immunogenicity of routine vaccines [22]. Noninferiority was established between the group of subjects receiving 4CMenB concomitantly with the 6-in-1 and PCV7 vaccines at the accelerated times of 2, 3, and 4 months and the group who received only routine vaccinations at these timepoints in terms of the seroresponse rates to all but the pertactin component of 6-in-1 and all but the pneumococcal serotype PnC 6B component of PCV7; the threshold of response differed for all vaccine antigens and were those accepted by regulatory authorities. Noninferiority was established when the lower limit of the 95 % confidence interval for the difference in seroresponse rates between the two groups (accelerated concomitant minus control) for each 6-in-1 or PCV7 antigen was greater than −10 %.
2.2 In Adolescents and Adults
Two doses of 4CMenB vaccine also elicited a strong immune response in adolescents [23] and adults [24].
2.2.1 In Adolescents
At baseline, seroprotective titers against fHbp, NadA, or PorA were seen in 44 %, 34 %, and 35 % of adolescents, respectively, with these high rates potentially being caused by nasopharyngeal carriage and a high level of environmental exposure to meningococcal serogroup B strains [23].
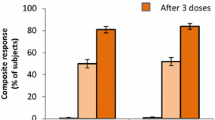
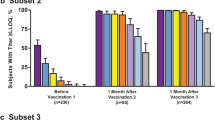
One month after one dose of 4CMenB (administered at month 0, 1, 2, or 6), overall seroprotection rates against fHbp, NadA, or PorA were 93 %, 96 %, and 93 %, respectively, regardless of baseline serostatus. One month after a second dose (administered at month 1, 2, or 6), seroprotection rates were significantly (p < 0.0001) higher than after one dose, reaching >99 % for all of the test strains [23]. A third dose of 4CMenB (administered in month 2 or 6) did not appear to offer any additional immunogenic advantage over two doses, with 99–100 % of adolescents who received three doses achieving seroprotective hSBA titers against each of the three test strains [23].
In subjects who received only one dose of 4CMenB in phase one of the study, seroprotective titers against the three test strains persisted until 6 months in 69–81 % of adolescents and, in those who received two or three doses in phase one, seroprotective rates at 6 months were 89–100 % [23].
In the group of subjects who received a single dose of 4CMenB at month 0 and then a booster dose at month 6, seroprotection rates increased from 76–81 % at month 6 to 99–100 % at month 7 [23].
The post-vaccination seroprotection rate against each of the three test strains was 29–50 % in subjects who received only placebo in phase one of the study, again potentially reflecting the high rate of nasopharyngeal carriage of meningococcal serogroup B strains and of environmental exposure to these bacteria [23].
2.2.2 In Adults at a Potentially Increased Risk of Meningococcal Exposure
Immune responses to fHbp, NadA, and PorA were elicited after one dose of 4CMenB, with 80–88 % (versus 22–37 % at baseline) of subjects achieving seroprotective hSBA titers 1 month after dose administration; the immune response to one dose of 4CMenB was higher than expected, indicating that these patients may have been previously primed through handling of meningococcal isolates in the work environment [24]. After two doses of 4CMenB, seroprotection rates had risen to 91–100 % and, after three doses, they were 92–100 %.
In subjects with no detectable bactericidal titers at baseline (i.e. hSBA titers of <4 against at least one of the test strains), 89 % achieved seroprotective titers after two doses of 4CMenB and 90 % after three doses [24].
2.3 Cross-Reactivity and Coverage
The potential coverage of 4CMenB against meningococcal serogroup B strains is broad, according to results of a preclinical study primarily investigating a new method (the Meningococcal Antigen Typing System [MATS]) of defining antigen phenotypes that confer susceptibility to vaccine-induced bactericidal activity [15]. The study demonstrated that MATS (which is based on the extent of immunologic recognition and the level of antigen expression) could give a conservative estimate of the 4CMenB-induced killing rates of meningococcal serogroup B strains in the serum bactericidal assay (SBA).
In sera obtained from subjects who had previously been vaccinated with 4CMenB (i.e. immune sera), the MATS predicted coverage of 4CMenB against a panel of meningococcal serogroup B strains (n = 57–124) that were obtained from various countries was 77 % in pooled sera from infants, 70 % in pooled sera from adolescents, and 72 % in pooled sera from adults [15]. Of note, the actual killing rate in the SBA was 74 %, 67 %, and 85 % in immune sera from infants, adolescents, and adults, respectively.
Although each antigen contained in 4CMenB can independently induce bactericidal antibodies against meningococcal serogroup B strains, killing rates were higher in bacterial strains that were positive for multiple vaccine antigens [15]. For example, in serum pools obtained from adults who had previously received 4CMenB, killing by 4CMenB-induced bactericidal antibodies occurred in 85 %, 94 %, and 100 % of the meningococcal serogroup B strains that were positive for one (n = 41), two (n = 34), or three (n = 16) of the vaccine antigens, respectively.
4CMenB has the potential to protect against a large proportion of invasive disease-causing meningococcal serogroup B strains in Europe, according to another study that used MATS to evaluate strain coverage [27]. Of 1,052 invasive disease-causing meningococcal serogroup B strains obtained from national reference laboratories across Europe (isolated between July 2007 and June 2008), 4CMenB had the potential to provide cover for an estimated 78 % of strains overall, and 73–87 % of strains when stratified by country [27]. In addition, 4CMenB had the potential to induce bactericidal antibodies against more than one antigen in half of all strains.
3 Reactogenicity
The reactogenicity of 4CMenB in infants [21, 22, 25, 28], adolescents [23], or adults [24] was assessed in the clinical studies discussed in Sect. 2. The primary phase III study in infants was conducted in two parts: an open-label immunogenicity substudy (discussed in Sect. 2.1.1.1) and an observer-blind safety substudy [21]. Infants in the safety substudy were randomized to receive one of three lots of 4CMenB plus 6-in-1 and PCV7 at 2, 4, and 6 months or 6-in-1, PCV7, and the serogroup C meningococcal conjugate vaccine (MenC) at 2, 4, and 6 months [21]. Because reactogenicity data from the immunogenicity and safety substudies were similar, data from the primary phase III infant study are presented as pooled results from both substudies (n = 3,626–3,627 infants in safety population) [21].
The reactogenicity of 4CMenB was generally acceptable, with most adverse reactions being mild to moderate in severity [21–25].
Injection-site tenderness/pain (≈55–98 % of subjects [22, 24, 25]; 86 % of injections [23]), erythema (≈41–70 %; 50 %), and induration (≈40–50 %; 40 %) were the most commonly reported solicited local reactions occurring within 7 days of the first 4CMenB dose in infants [22, 25], adolescents [23], and adults [24] (some values estimated from graphs).
The most frequent solicited systemic reactions occurring within 7 days of the first 4CMenB dose were irritability (≈60–79 % of subjects), sleepiness (≈40–73 %), change in appetite (≈32–59 %), and unusual crying (28–68 %) in infants [22, 25], and malaise (≈30 % [24]; 51 % of injections [23]), myalgia (≈28 %; 42 %), headache (≈28 %; 42 %), arthralgia (≈19 %; 25 %), and nausea (≈12 %; 16 %) in adolescents [23] and adults [24] (some values estimated from graphs).
3.1 In Infants
3.1.1 Fever
3.1.1.1 Compared with the 6-in-1 and PCV7 Vaccines
In general, fever appeared to occur more frequently in infants who received 4CMenB concomitantly with 6-in-1 and PCV7 than in those who received 4CMenB [22] or 6-in-1 and PCV7 [21] alone.
In the primary phase III trial, the incidence of fever ≥38.5 °C occurring within 6 h of administration of any of the three vaccine doses was ≈2-fold more common in infants aged 2–6 months who received 4CMenB concomitantly with the 6-in-1 and PCV7 vaccines than in those who received 6-in-1 and PCV7 alone or concomitantly with MenC (65.3 % vs. 32.2 % and 33.7 %) [21]. Fever ≥40 °C occurring within 6 h of vaccination was seen in 1.2 % of infants who received 4CMenB concomitantly with 6-in-1 and PCV7, 0 % of those who received 6-in-1 and PCV7 alone, and 0.2 % of those who received 6-in-1 and PCV7 concomitantly with MenC. Antipyretic agents were required in 93 % of infants who received 4CMenB concomitantly with 6-in-1 and PCV7, 71 % of those who received 6-in-1 and PCV7 alone, and 66 % of those who received 6-in-1 and PCV7 concomitantly with MenC. The incidence of medically-attended fever was low, occurring in ≤3 % of infants in any of the treatment groups [21]. In this trial and in the subsequent booster dose trial, peak temperatures occurred within the first 6 h after vaccination, which dropped by the following day and had returned to background levels by day 3 [21].
During the first 7 days after administration of the first vaccine dose in the phase IIb study, fever ≥38 °C or ≥39 °C occurred in 38 % and 7 % of 2-month-old infants who received 4CMenB alone and in 58–61 % and 11–12 % of those who received 4CMenB concomitantly with the 6-in-1 and PCV7 vaccines [22]; the incidence of fever ≥38 °C and ≥39 °C in infants who received the 6-in-1 and PCV7 vaccines alone was 31 % and 4 %. In 95 % of infants, fever resolved within 24–48 h of vaccine administration [29]. Six infants in the phase IIb trial (five of whom received 4CMenB [either with or without the 6-in-1 and PCV7 vaccines] and one who received the 6-in-1 and PCV7 vaccines alone) were hospitalized because of fever within 2 days of vaccine administration [22].
3.1.1.2 Compared with the MMRV Vaccine
In 12-month-old infants who had received a booster dose of 4CMenB with or without MMRV, fever ≥38 °C was seen in 31–32 % of subjects within 6 h of 4CMenB vaccination [21]. As is characteristic of MMRV vaccines, a second temperature peak was seen around day 9 in infants who received concomitant 4CMenB and MMRV [21]. Fever ≥40 °C was rare in the booster dose study, occurring within 6 h of receiving concomitant 4CMenB and MMRV in two infants and during the second MMRV-associated temperature peak around day 9 in six infants; in the 4 days following the booster dose, there were no cases of fever ≥40 °C in infants who received 4CMenB alone [21].
Fever ≥38 °C was seen in 35–40 % of 12-month-old infants after administration of a first catch-up dose of 4CMenB with or without concomitant MMRV during the first 4 days after vaccination and in 8 % of those who received MMRV alone [25]. However, during the 5–28 days post-vaccination, fever was reported in 44 % of subjects who received the vaccines together and in 53 % of those who received MMRV alone [25].
3.1.1.3 Serious Fever-Related Adverse Reactions
Febrile seizures occurred in six infants who received 4CMenB with 6-in-1 and PCV7 in the primary phase III trial, two of which occurred after the second vaccine dose and were thought probably related to 4CMenB [21]; the other four episodes occurred after administration of the third vaccine dose and were considered related to underlying infection. Two other cases of (mild or moderate) seizures occurred in the presence of fever after the first vaccine dose (coadministered with 6-in-1 and PCV7) and were thought possibly related to 4CMenB [21]. One possibly treatment-related febrile seizure also occurred in a recipient of 4CMenB in the phase IIb study [22]. None of the episodes of febrile seizures that occurred in the catch-up [25] or booster [21] studies were thought to be related to 4CMenB; however, one episode of febrile seizure occurring 9 days after coadministration of 4CMenB with MMRV in the booster study was thought possibly related to MMRV [21]. One other possibly vaccine-related serious adverse event occurred in the booster dose study, an episode of fever occurring after coadministration of 4CMenB and MMRV [21].
3.1.2 Other Solicited Systemic Reactions
Solicited systemic reactions appeared to have a numerically higher incidence in 2- to 6-month-old infants who received 4CMenB concomitantly with 6-in-1 and PCV7 than in those who received the 6-in-1 and PCV7 vaccines alone or concomitantly with MenC [21]. Irritability, sleepiness, unusual crying, or changes in feeding occurred in 93 %, 87 %, 85 %, and 72 % of infants after receiving any of three doses of 4CMenB (administered with the 6-in-1 and PCV7 vaccines), in 83 %, 72 %, 64 %, and 50 % of those who received the 6-in-1 and PCV7 vaccines alone, and in 76 %, 72 %, 72 %, and 52 % of those who received 6-in-1 and PCV7 concomitantly with MenC [21].
Coadministration of 4CMenB with (as opposed to without) MMRV was also associated with a small increase (≤1.5-fold) in the incidence of solicited systemic reactions after the first vaccine dose in the catch-up trial [25].
The incidence of solicited systemic reactions appeared to be lower after a booster dose of 4CMenB (with or without MMRV) in infants aged 12 months than after the primary series of three doses of 4CMenB (with 6-in-1 and PCV7) in infants aged 2–6 months [21]. For example, irritability occurred in 68–73 % infants after the booster dose and in 93 % of infants after any of the primary series of doses.
3.1.3 Solicited Local Reactions
4CMenB also appeared to be associated with a numerically higher incidence of solicited local reactions than the 6-in-1 and/or PCV7 vaccine in young infants [28]. For example, after administration of the first dose of 4CMenB or the 6-in-1 vaccine to infants aged 2 months in a phase IIb trial, erythema occurred in 62–66 % and 47–55 %, tenderness in 58–65 % and 38–55 %, and swelling in 24–31 % and 14–18 % of infants (values estimated from graphs) [28].
In the three groups (i.e. infants receiving three doses of 4CMenB concomitantly with 6-in-1 and PCV7; 6-in-1 and PCV7 alone; or 6-in-1 and PCV7 concomitantly with MenC) of the primary phase III trial, local tenderness occurred at any of the injection sites in 79–87 %, 53–59 %, and 54–68 %, respectively, erythema in 70–83 %, 62–71 %, and 53–74 %, induration in 55–77 %, 49–64 %, and 46–75 %, and swelling in 32–47 %, 27–34 %, and 17–30 % of infants aged 2–6 months [21].
3.1.4 Other Adverse Reactions
The most frequently occurring severe adverse reaction associated with 4CMenB was severe solicited local tenderness [21, 25, 28]. For example, in the primary phase III study, severe local tenderness (defined as crying on movement of limb) occurred in 24–29 % of infants receiving 4CMenB concomitantly with 6-in-1 and PCV7 [21]. In comparison, the incidence of severe solicited local tenderness reactions was 5–10 % after administration of the 6-in-1 and PCV7 vaccines alone or concomitantly with MenC [21].
In the primary phase III study, serious adverse events occurred in 8 % of infants receiving 4CMenB concomitantly with 6-in-1 and PCV7, 8 % of infants who received 6-in-1 and PCV7 alone, and 6 % who received 6-in-1 and PCV7 concomitantly with MenC [21]. Seventeen serious adverse events (16 in 4CMenB-receiving recipients and one in a recipient of routine vaccines only) were thought to be vaccine-related. Two confirmed cases and one unconfirmed case of Kawasaki disease occurred in 4CMenB-receiving infants in the primary phase III study [21]. No episodes of Kawasaki disease occurred in the booster dose study [21] and the one suspected case to occur in the catch-up dose study was not thought to be 4CMenB-related [25].
In the phase IIb study, other (i.e. nonfever-related) serious adverse reactions reported were: three episodes of seizures, which were thought to be possibly-related to study vaccine (one following 4CMenB administration in one of the concomitant treatment arms and two following administration of routine vaccines); two hypotonic, hypo-responsive episodes (one after concomitant administration of 4CMenB with routine vaccines and one after routine vaccines alone); and two cases of Kawasaki disease (one of which was thought to be possibly related to 4CMenB) [22].
Where mentioned, no study discontinuations occurred because of serious adverse events in infants participating in the 4CMenB clinical trials [25].
3.2 In Adolescents
The incidence of adverse events appeared to be similar in adolescents who received a two-dose schedule of 4CMenB (2 months apart) and those who received a three-dose schedule (1 month apart) after both the first (93 % and 95 %) and second doses (90 % and 91 %) [23]; in adolescents who received three doses of 4CMenB, the incidence of adverse events was 87 % after the third dose.
The incidence of fever ≥38 °C was less common in adolescents than in infants, being reported after 4 % of 4CMenB injections [23]. Fever ≥39 °C occurred in 1 % of adolescents.
Two cases of juvenile arthritis were reported 170 and 198 days after administration of a third dose of 4CMenB, one of which was considered probably related to study vaccine and the other possibly related [23]. There were no other study vaccine-related serious adverse events reported in the adolescent study.
3.3 In Adults at a Potentially Increased Risk of Meningococcal Exposure
In healthy adults at a potentially increased risk of meningococcal exposure, ≈98 %, ≈42 %, and ≈41 % reported injection-site pain, induration, or erythema after the first 4CMenB dose and ≈30 %, ≈28 %, ≈28 %, ≈19 %, and ≈16 % reported malaise, myalgia, headache, arthralgia, or nausea (values estimated from a graph). Most solicited reactions were transient, resolving within 3 days of vaccination [24].
It appeared that there was a numerically higher incidence of adverse reactions, particularly local reactions, after administration of 4CMenB than after administration of the meningococcal conjugate vaccine against serogroups A, C, W135, and Y (MenACWY-CRM) in this study [24]. For example, the incidence of injection-site pain, induration, or erythema was 4-, 3.5-, and 2-fold higher in 4CMenB versus MenACWY-CRM recipients, respectively (values estimated from a graph).
Fever was reported in 3 subjects, 4–5 subjects needed to stay at home after each vaccine dose, and 5–10 subjects took antipyretic or analgesic therapy after 4CMenB administration [24]. No serious adverse events or deaths occurred in this study [24].
4 Dosage and Administration
4CMenB is approved in the EU for active immunization against disease caused by N. meningitidis serogroup B in individuals aged ≥2 months [18]. In infants, the recommended vaccination schedule is three once-monthly doses of 4CMenB starting at 2 months of age followed by a booster dose between 12–23 months of age. In all other patient groups (i.e. unvaccinated infants aged ≥6 to 11 months, unvaccinated children aged 12–23 months, children aged 2–10 years, adolescents aged ≥11 years, and adults), the recommended vaccination schedule is two doses administered 1 (adults and adolescents) or 2 (unvaccinated infants and children) months apart. Unvaccinated infants aged 6–11 months of age should receive a booster dose in their second year of life, with an interval of ≥2 months between the booster dose and the primary series. Unvaccinated children aged 12–23 months should also receive a booster dose 12–23 months after the primary series. It is not yet known if or when additional booster doses may be required in infants or children receiving the primary series before 23 months of age. No booster dose is recommended in children aged 2–10 years, adolescents, or adults.
4CMenB is available in a prefilled syringe, with each dose (0.5 mL) containing 50 μg of the purified N. meningitidis antigens NadA, fHbp (fused with GNA2091), and NHBA (fused with GNA1030), and 25 μg of OMVs from N. meningitidis NZ98/254 [18]. The vaccine should be administered intramuscularly into the anterolateral aspect of the thigh (in infants) or the deltoid muscle (in all other patient groups), with separate injection sites being used if the vaccine is coadministered with other vaccines.
4CMenB may be coadministered with one or more of the following (monovalent or combination) vaccines: acellular pertussis, diphtheria, H. influenzae type b, hepatitis B, heptavalent pneumococcal conjugate, inactivated poliomyelitis, measles, mumps, rubella, tetanus, and varicella [18]. However, separate vaccination times may be considered, where appropriate, because of the increased risk of adverse events seen when 4CMenB was coadministered with one or more of these vaccines (Sect. 3.1).
4CMenB is associated with a higher risk of fever in infants when coadministered with 6-in-1 and PCV7 than when these vaccines are administered alone (Sect. 3.1.1). To reduce the incidence and intensity of febrile reactions, antipyretic agents may be administered, in accordance with local guidelines, at the time of 4CMenB vaccination and again after vaccination in infants and young children aged <2 years [18]. The vaccine should not be administered in patients with a severe acute febrile illness, although it may be administered in patients with minor infections (e.g. colds).
Infants born very prematurely (i.e. ≤28 weeks gestation), particularly those with respiratory immaturity, may be at increased risk of apnea requiring respiratory monitoring for 48–72 h following the primary series of 4CMenB vaccination at age 2–5 months [18]. Because of this, it is important to weigh up the benefits versus the risks of vaccination in such infants. However, the benefit of receiving 4CMenB is high in this group of patients.
There are no data pertaining to the efficacy of 4CMenB in adults aged >50 years, patients with impaired immune responsiveness, or in patients with chronic medical conditions; 4CMenB may not provide a protective antibody response in immunocompromised patients [18].
Local prescribing information should be consulted for additional information regarding warnings, precautions, contraindications, and use in special patient populations.
5 Multicomponent Meningococcal Serogroup B Vaccine (4CMenB; Bexsero®): Current Status
4CMenB is approved in the EU for active immunization against disease caused by N. meningitidis serogroup B in individuals aged ≥2 months.
In phase IIb or III trials, three doses of 4CMenB administered to young infants (aged ≤6 months) elicited seroprotective hSBA antibody titers against meningococcal serogroup B test strains that were specific for vaccine antigens in the majority of subjects. Similar results were seen when two catch-up doses (vaccine-naive infants) or a single booster dose (vaccine-experienced infants) of 4CMenB was administered to 12-month-old infants in extensions to the phase III trial.
The majority of adolescents participating in a phase IIb/III trial achieved seroprotective hSBA antibody titers against all test strains after two doses, and a third dose did not appear to give any additional immunogenic advantage. Two or three doses of 4CMenB elicited seroprotective hSBA antibody titers against all test strains in the majority of adults who were at a potentially increased risk of meningococcal exposure in a small phase II study.
Further large phase III studies assessing the immunogenicity of 4CMenB in adolescents and adults are required, as are trials evaluating the protective efficacy of the vaccine in all age groups. The reactogenicity of 4CMenB was generally acceptable. However, in infants, the vaccine was associated with a numerically higher incidence of solicited systemic adverse events (particularly fever) when coadministered with 6-in-1 and PCV7 than when these vaccines were administered alone.
References
National Foundation for Infectious Diseases. The changing epidemiology of meningococcal disease among U.S. children, adolescents and young adults. 2004. http://www.nfid.org/pdf/meningitis/FINALChanging_Epidemiology_of_Meningococcal_Disease.pdf. Accessed 11 Sep 2011.
Caugant DA, Maiden MCJ. Meningococcal carriage and disease: population biology and evolution. Vaccine. 2009;27(4):B64–70.
European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe. 2008/09. http://ecdc.europa.eu/en/publications/Publications/1107_SUR_IBD_2008-09.pdf. Accessed 4 Mar 2013.
Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med. 2001;344(18):1378–88.
Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–210.
Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007;31(1):101–7.
Novartis Vaccines. Meningococcal group C conjugate vaccine (Menjugate Kit®): UK prescribing information. 2012. http://www.medicines.org.uk/EMC/medicine/16597/SPC/Menjugate+Kit/. Accessed 4 Mar 2013.
Pfizer Limited. Meningitec® in pre-filled syringe: UK prescribing information. 2011. http://www.medicines.org.uk/EMC/medicine/20747/SPC/Meningitec+in+pre-filled+syringe/. Accessed 4 Mar 2013.
Novartis Vaccines and Diagnostics. Meningococcal group A, C, W135, and Y conjugate vaccine (Menveo®): EU prescribing information. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001095/WC500090147.pdf. Accessed 4 Mar 2013.
EUVAC.net. Meningococcal vaccination (MenC) overview in European countries. http://www.euvac.net/graphics/euvac/vaccination/menc.html. Accessed 4 Mar 2013.
Gray SJ, Trotter CL, Ramsay ME, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the meningococcal reference unit. J Med Microbiol. 2006;55:887–96.
Cohn AC, Harrison LH. Meningococcal vaccines: current issues and future strategies. Drugs 2013. In press.
Granoff DM. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50(Suppl 2):S54–65.
Lucidarme J, Comanducci M, Findlow J, et al. Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. J Clin Microbiol. 2009;47(11):3577–85.
Donnelly J, Medini D, Boccadifuoco G, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci USA. 2010;107(45):19490–5.
Pizza M, Scarlato V, Masignani V, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287(5459):1816–20.
Fletcher LD, Bernfield L, Barniak V, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72(4):2088–100.
Novartis Vaccines and Diagnostics. Meningococcal group B vaccine (Bexsero®) suspension for injection: EU summary of product characteristics. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002333/WC500137881.pdf. Accessed 13 Feb 2013.
Findlow J, Borrow R, Snape MD, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51(10):1127–37.
Snape MD, Dawson T, Oster P, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J. 2010;29(11):e71–9.
Vesikari T, Esposito S, Prymula R, et al. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomized trials. Lancet. 2013. doi:10.1016/S0140-6736(12)61961-8.
Gossger N, Snape MD, Yu L-M, et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307(6):573–82.
Santolaya ME, O’Ryan ML, Valenzuela MT, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379(9816):617–24.
Kimura A, Toneatto D, Kleinschmidt A, et al. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin Vaccine Immunol. 2011;18(3):483–6.
Prymula R, Vesikari T, Esposito S, et al. Catch-up vaccination of healthy toddlers with an investigational multicomponent meningococcal serogroup B vaccine (4CMenB):exploration of a two-dose schedule [poster]. 29th European Society for Paediatric Infectious Diseases Conference; 7–11 June 2011; The Hague.
Vesikari T, Prymula R, Liese J, et al. Booster dose at 12 months of an investigational meningococcal serogroup B vaccine (4CMenB) in healthy toddlers previously primed at 2, 4, 6 months [poster]. 29th European Society for Paediatric Infectious Diseases Conference; 7–11 June 2011; The Hague.
Vogel U, Taha M-K, Vazquez JA, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013. doi:10.1016/S1473-3099(13)70006-9.
Beeretz I, MD S, A F, et al. Reactogenicity and safety of multicomponent meningococcal serogroup B vaccine (4CMenB) administered with or without routine infant vaccinations in different schedules [poster]. 29th European Society for Paediatric Infectious Diseases Conference; 7–11 June 2011; The Hague.
Novartis. Novartis candidate vaccine Bexsero® shows significant potential in providing broad coverage against meningococcal serogroup B infections. 2011. http://www.novartis.com/newsroom/media-releases/en/2011/1522283.shtml. Accessed 28 Mar 2013.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was also offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: J. Findlow, Healthy Protection Agency North West, Manchester Royal Infirmary, Manchester, UK; R. Prymula, Faculty of Military Health Sciences, Department of Epidemiology, Hradec Králové, Czech Republic; T. Vesikari, Department of Virology and Vaccine Research, University of Tampere Medical School, Tampere, Finland.
Rights and permissions
About this article
Cite this article
Carter, N.J. Multicomponent Meningococcal Serogroup B Vaccine (4CMenB; Bexsero®): A Review of its Use in Primary and Booster Vaccination. BioDrugs 27, 263–274 (2013). https://doi.org/10.1007/s40259-013-0029-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-013-0029-2