Abstract
Objectives
Invasive pneumococcal disease (IPD), pneumonia and acute otitis media (AOM) still represent a significant medical burden in children < 5 years of age in New Zealand (NZ), with marked disparities across socio-economic and ethnic groups. This cost-effectiveness evaluation aims to compare the potential impact of two childhood universal immunisation strategies: vaccination with a 3 + 1 schedule of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV, Synflorix, GSK) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevenar 13, Pfizer).
Methods
A static Markov-process cohort model was used to simulate the epidemiological and economic burden of pneumococcal diseases on a single-birth cohort over its lifetime. Costs and outcomes were discounted annually at 3.5%. Epidemiological and cost inputs were extracted from the most recently available NZ data, or derived from the most relevant reference countries’ sources. The most updated evidence on the efficacies of the corresponding vaccines were used, particularly the significant effectiveness for PHiD-CV against IPD caused by serotype 19A.
Results
The model estimated that both vaccines have a broadly comparable impact on IPD-related diseases and pneumonia. Due to the additional benefits possible through broader impact on AOM, PHiD-CV is estimated to potentially provide additional discounted cost offsets of approximately NZD 0.8 million over the lifetime of the birth cohort.
Conclusions
To ensure health equity in children, given the substantial burden of pneumonia and AOM, decision-makers should also take into account the impact of PCVs on these diseases for decisions relating to routine infant immunization.
GSK study identifier
HO-15-16775.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently published evidence from well-designed studies in countries around the world that have used PHiD-CV in their national immunisation programmes point to a significant level of protection against invasive pneumococcal diseases caused by pneumococcal serotype 19A. Real-world evidence also points to protection against serotype 6A. |
In light of the updated real-world evidence, both higher-valent pneumococcal conjugate vaccines (PHiD-CV and PCV13) were estimated to offer equivalent protection for infants against invasive pneumococcal diseases in New Zealand, in line with the findings by independent reviews conducted by PAHO and IVAC. |
When considering the additional protection potentially offered by PHiD-CV against middle-ear disease caused by non-typeable Haemophilus Influenzae, PHiD-CV was estimated to produce greater health benefits while also being the less costly option compared to PCV13, assuming equivalent acquisition costs. |
1 Introduction
Streptococcus pneumoniae (Spn) is a leading cause of acute otitis media (AOM), invasive pneumococcal disease (IPD) and pneumonia in children. Despite substantial reductions in IPD and pneumonia since the introduction of pneumococcal conjugate vaccines (PCVs), almost half a million deaths in children < 5 years old are still estimated to occur annually worldwide [1].
Spn may cause meningitis and bacteraemia, especially in children < 2 years of age, and is often the cause of bacteraemia with no obvious primary site of infection [2]. In New Zealand (NZ), IPD had a case-fatality ratio of 1.96% in the < 5-years age group in 2014 [3].
Otitis media (OM) is one of the most common disorders requiring medical care for children [4]. Spn, non-typeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis are considered the major causative pathogens responsible for OM. In NZ children < 36 months of age who underwent ventilation tube (grommet) surgery, NTHi was detected in 43.5% of the middle ear fluid samples of cases with recurrent AOM and OM with effusion (OME) and Spn was detected in 23% [4]. OM can be debilitating and, if untreated, can impact hearing and result in significant delays in learning and development [5, 6]. Prevention and prompt treatment of OM may help improve health equity. An analysis of NZ hospital admissions between 2000 and 2007 showed that children living in areas of higher socio-economic deprivation had twice the number of OM hospitalisations compared with children living in areas with the lowest socio-economic deprivation [7]. Furthermore, Maori and Pacific Island children with OM are less likely to have important surgical interventions, such as grommets [7, 8] and hence more likely to develop long-term sequelae.
Pacific children also had the highest childhood pneumonia rates, at 797 hospitalisations/100,000, which was 3.1 times higher than the rate in children of European descent [9]. The inequality in childhood pneumonia mortality also mirrored the hospitalisation rates. Socio-economic inequalities were even more marked with a clear trend of higher mortality with increasing socio-economic deprivation [9].
Since June 2008, vaccination with four doses of PCV has been fully funded in NZ for previously unvaccinated individuals up to the age of 59 months. The 7-valent PCV (PCV7, Prevenar, Pfizer) was the first conjugate vaccine funded in NZ. The 10-valent pneumococcal NTHi protein D conjugate vaccine (PHiD-CV, Synflorix, GSK) replaced PCV7 in October 2011. The 13-valent PCV (PCV13, Prevenar 13, Pfizer) replaced PHiD-CV from October 2014 onwards [10] and the latter replaced the former from July 2017 [11].
PCVs have had a significant impact on IPD in NZ. In children < 5 years of age, the rate of IPD due to the 10 serotypes included in PHiD-CV decreased by 95.6% (from 44.2/100,000 to 1.9/100,000 population from 2006 to 2014) [3]. The overall rate of IPD, irrespective of serotype, decreased by 66.7% (53.5–17.8/100,000) over the same period [3]. Due to the indirect effects of routine infant PCV immunisation, there have also been 60 and 70% reductions in IPD due to PHiD-CV serotypes in the 5–64-years and ≥ 65-years age groups, respectively, between 2006 and 2014 [3].
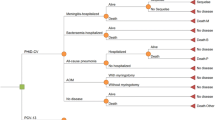
The objective of this cost-effectiveness evaluation is to estimate and compare the potential impact of two childhood universal immunisation strategies in NZ: routine vaccination of infants with a 3 + 1 schedule of PHiD-CV or PCV13 (Fig. 1).
2 Methods
2.1 Model Description
A previously published static Markov cohort model [12] (Fig. 2) implemented in Microsoft Office Excel (2007) was adapted to estimate and compare the accumulated outcomes of a single-birth cohort over a lifetime, using a monthly time-cycle, under two steady-state scenarios in the base-case: vaccinated with a 3 + 1 schedule (defined as a one primary dose each at months 2, 3 and 5 and a booster dose at 15 months of age) of either PHiD-CV or PCV13. The model structure and underlying assumptions were further validated using an expert panel (Leuven, September 2013). In the base-case, costs (from a payer perspective) and outcomes were discounted at 3.5% as per the NZ Pharmaceutical Management Agency (PHARMAC) cost-utility guidelines [13].
2.2 Demographics and Epidemiology
The size of the 2014 birth cohort for NZ (57,242) and age-specific overall mortality rates were obtained from national databases [14, 15].
Age-specific incidence rates of IPD were obtained from the annual Institute of Environmental Science and Research Ltd (ESR) surveillance report for 2014—the most recently available report for NZ (Supplementary Table 1) [3]. Cases of empyema, pneumonia, bacteraemia and other IPD were grouped as ‘other IPD’. The age-specific serotype distribution of IPD cases was sourced from the 2014 ESR annual report (Supplementary Table 2) [3]. Age-specific case-fatality ratios (CFR) were calculated from the same report [3].
For IPD meningitis, the proportions of neurological sequelae and severe hearing loss were estimated based on Morrow et al. [16]. Due to lack of specific epidemiological data on sequelae following other IPD, no sequelae were assumed for these cases.
All-cause pneumonia hospitalisation rates (Supplementary Table 1) were taken from Milne and Vander Hoorn for children ≤ 5 years as the rates were presented by single-year age groups [7]. For children > 5 years, data were obtained from the publicly funded hospital discharges from July 2012 to June 2013 data for ICD-10 codes J12-J18 (the most recent data available) [17]. Age-specific CFRs were based on reported deaths in 2012 for ICD-10 codes J12-J18 [18]. General practitioner (GP) consultations were estimated as a ratio of 1:1 to hospitalisations based on the observations recorded in the UK using Hospital Episode Statistics (HES) database for 2013–2014 [19] as well as a cross-sectional analysis of the Medical Expenditure Panel Survey (MEPS) database between 2007 and 2011 in the USA [20].
AOM incidence rates (Supplementary Table 1) in primary-care settings for the < 5-years age group were sourced from Gribben et al. [21]. Since NZ-specific data for children ≥ 5 years were not available, GP consultation rates from the UK were used [22]. It was assumed that there were no AOM consultations beyond the age of 14 years. The number of myringotomy procedures performed in NZ was obtained from the publicy-funded hospital discharges and procedures database of the Ministry of Health (MoH) using ICD-10 code 309 [17]. For the proportion of AOM cases with complications, hospital discharges for ICD-10 codes H65 (Non-suppurative otitis media) and H66 (Suppurative and unspecified otitis media) were used [17].
2.3 Vaccine Effectiveness
The model assumed that vaccine efficacy (VE) increased with the increasing number of doses until the maximum efficacy was achieved after the booster dose based on the observed effectiveness values observed with PCV7 [23]. The model assumed that protection persisted up to 3 years of age for the 3 + 1 regimen. A long-term efficacy study for the 9-valent PCV conducted in South African children observed that VE against vaccine-serotype IPD following 6.16 years of follow-up persisted in HIV non-infected children (77.8% compared to 83% after 2.3 years of follow-up) [24]. Additionally, a meta-regression analysis of PCV VE against nasopharyngeal carriage (NPC) estimated a 23.6% reduction in VE against carriage of PCV7 serotypes 5 years following the completion of vaccination [25]. Based on these evidences, VE in the current model was assumed to, conservatively, decline exponentially up to the age of 10 years.
2.4 IPD Effectiveness
The overall effectiveness of each vaccine against IPD is a function of the age-specific serotype distribution and the serotype-specific efficacy (Table 1). A post-marketing matched case–control study demonstrated that PCV7, on average, was 94.7% effective against vaccine serotypes while also offering significant protection of 76% (95% confidential interval [CI]: 39–90) against serotype 6A [23]. Both PHiD-CV and PCV13 were licensed based on non-inferiority immunogenicity trials versus PCV7 [26,27,28], so serotype-specific efficacy of both against IPD caused by the seven common serotypes contained in all three vaccines, and the three additional common to both PHiD-CV and PCV13 were assumed to be 94.7%.
Despite the introduction of PCV13 into many national immunisation programmes (NIP) for several years, there is a wide range of estimated effectiveness against IPD caused by serotype 3 [29,30,31,32,33,34,35]. We assumed a 26% base-case efficacy for PCV13 in the model [29] while assessing values of 0% and 79.5% in the sensitivity analysis [34].
2.5 Protection Against 6A and 19A for PHiD-CV
Despite both PCV7 and PHiD-CV containing serotype 19F, Poolman et al. observed that PHiD-CV induced higher levels of functional antibodies against 19F and 19A than PCV7. They postulated that the processes (reductive animation vs. cyanylation) used to conjugate the polysaccharides to the corresponding vaccine carrier proteins could possibly explain the variation in immunological responses [36]. Effectiveness studies performed in various countries point to significant protection for PHiD-CV against IPD caused by 6A and 19A. A matched case–control study performed in Brazil following the introduction of PHiD-CV into the NIP showed an effectiveness of 82% (95% CI 10.7–96.4) against 19A IPD [37]. Verani et al. estimated an effectiveness of 71.3% (95% CI 16.6–90.1) [38] using the indirect cohort method on the same Brazilian data. These results from Brazil have been corroborated by additional studies from Canada, 71% (95% CI 24–89) [39], and Finland, 62% (95% CI 20–85) [40]. As the average value of the effectiveness results fell close to the estimate obtained by Verani et al., we assumed an effectiveness of 71.3% for PHiD-CV against 19A IPD.
For 6A IPD, a value of 76% was used based on the efficacy estimated for PCV7 [23].
2.6 All-Cause Pneumonia Effectiveness
The model uses VE against WHO-defined X-Ray confirmed Community-Acquired Pneumonia (WHO-CAP) as a surrogate measure of efficacy against hospitalised pneumonia. VEs of PCVs against WHO-CAP and suspected-CAP have been assessed in several large-scale, randomised trials [41,42,43,44]. Despite differences in study design, setting and vaccine formulation, efficacies were between 20–35 and 6–8%, for WHO-CAP and suspected-CAP, respectively.
A recent systematic review on the effectiveness of PHiD-CV and PCV13 on hospitalisations due to pneumonia in Latin American and Caribbean countries found no evidence of the superiority of one vaccine over the other in children < 5 years old [45]. In the absence of PCV13-specific pneumonia VE clinical trials in children, estimates of 21.8% (95% CI 7.7–33.7) for inpatient pneumonia and 8.7% (95% CI 3.8–13.4) for outpatient pneumonia were used for both vaccines based on the Clinical Otitis Media and PneumoniA Study (COMPAS, NCT00466947) [44].
2.7 AOM Effectiveness
VE against AOM is estimated based on the corresponding efficacies against pneumococcal vaccine serotypes, non-vaccine serotypes and AOM caused by NTHi (Table 1).
The model assumed that 35.9% of the AOM cases were attributable to Spn and 32.3% to NTHi [46]. The percentage of Spn AOM cases caused by vaccine serotypes is assumed to be 78.2% (assuming protection against 6A and 19A) [47].
VE against clinically-confirmed AOM (C-AOM) was obtained from COMPAS [44]: 69.9% (95% CI 29.8–87.1) against vaccine-type serotypes and 29.9% (95% CI − 123.7 to 77.5) against cross-reactive serotypes. A − 33% (95% CI – 80 to 1) efficacy was assumed for non-vaccine serotypes to account for serotype replacement [48].
Although the COMPAS trial was not powered to provide conclusive evidence for PHiD-CV efficacy against NTHi AOM, the positive point estimate of 21.5% (95% CI − 43.4 to 57) [44] is consistent with the statistically significant efficacy observed with the predecessor protein D conjugate formulation in the Pneumococcal Otitis Efficacy Trial (POET) study (35.3%; 95% CI 1.8–57.4) [49].
Black et al. reported an efficacy of 20.1% in preventing myringotomies and an efficacy of 7.0% in preventing AOM for PCV7 [50], implying that PCV7 was 2.87 times more effective at preventing myringotomy than AOM episodes. This ratio was used to estimate the reduction in myringotomy for each vaccine.
2.8 Herd Effects
Pneumococcal vaccination has been consistently followed by significant decreases in both vaccine-type (VT) carriage and VT-IPD in unvaccinated groups [52]. However, the benefits from reduction in VT-disease also have to be considered in the context of serotype replacement.
Jokinen et al. showed that in the Finnish Invasive Pneumococcal disease (FinIP) vaccine trial, PHiD-CV demonstrated an efficacy against VT-NPC of 29% (95% CI 6–47) in the siblings (aged 3–7 years) of the vaccinated children [53]. A population-based observational study in Finland also reported a 48% (95% CI 18–69) reduction in IPD among unvaccinated children aged 2–5 years with PHiD-CV [40]. Additionally, surveillance data from a number of countries using PHiD-CV clearly demonstrate VT herd effects in all age groups of older adults following the introduction of childhood vaccination programmes [54,55,56].
Given the complexity of accurately predicting the extent of herd protection, in the base-case analysis no indirect effect was applied, whereas net indirect effect was applied only to IPD in a scenario analysis. In the steady state, an indirect protection (corrected for serotype replacement) of 30% for PHiD-CV and 32% for PCV13 [57] was assumed.
2.9 Quality of Life
Normative utility values for the whole population were based on EQ-5D index value for NZ [58]. Annualised disutility values reported in a UK cost-effectiveness analysis of PCV7 based on several reported studies were used in the model (Table 2) [59].
2.10 Costs
All costs are expressed in 2015 NZ Dollars (NZD).
In line with the PHARMAC guidelines for pharmaco-economic analysis [13] and the cost resource manual [64], hospital inpatient costs were estimated using Diagnostic Related Group (DRG) prices. For each DRG code, the corresponding inlier multiday weight was obtained from the weighted inlier separations dataset for 2015/16 published by the MoH [65]. The PHARMAC cost resource manual for 2015 states a unit price of NZD 4751.58 [64]. Data on the discharges for each DRG code for 2012/13 were obtained from the MoH. The weighted average costs for each health state was subsequently estimated (Table 3 and Supplementary Table 3).
IPD and pneumonia outpatient costs are assumed to be NZD 300 each (initial outpatient physician consultation costs for 2015) while AOM GP visits are assumed to cost NZD 75 [64]. An AOM or pneumonia event is assumed to require one consultation only.
Milne et al. estimated the long-term costs associated with survivors of paediatric pneumococcal meningitis who had severe disability [66]. The net present value (NPV) longitudinal cost (inflated to 2015 NZD) from 12 months to 64 years of age was obtained from the total of age-specific disability funding discounted annually at 3.5%. An age-independent annual cost was calculated by estimating the yearly payments that would result in the same NPV after 65 years discounted at 3.5% (assuming an annuity costing methodology). The estimated annual cost was NZD 21,331 (Supplementary Table 4).
Price-parity per dose is assumed for PHiD-CV and PCV13 with an administration cost (per dose) of NZD 22.93 [64]. While the vaccine price chosen for each vaccine will significantly affect the outcomes and acknowledging that the final price in an NZ universal mass vaccination setting is a result of a complex tendering process, price-parity was assumed to focus on the clinical differences between both vaccines.
Both vaccines are considered to have an acceptable safety profile and serious adverse events (SAE) are estimated to be rare [67, 68]. Hence, we have not considered the impact of SAEs in our analysis.
Indirect costs are considered in a scenario analysis. The 2015 median income data was obtained from the NZ statistics website [69]. It was assumed that only 20–49-year-olds will be affected in terms of indirect costs and the average of the median monthly income is estimated (NZD 3286). It was further assumed that the time taken off work is equal to the average length of stay (ALoS) for the outcome as obtained from the NZ MoH weighted inlier separations dataset for 2015/2016 (Table 3) [65].
2.11 Sensitivity Analyses
A one-way sensitivity analysis was performed to explore the individual impact of uncertainties in model parameters on the model results. The sensitivity analysis was performed using pre-defined ranges or published 95% CI values for each parameter (Supplementary Table 5). A multivariate sensitivity analysis was also performed using a Monte Carlo simulation over 1000 iterations and the results are presented on the cost-effectiveness plane.
3 Results
3.1 Base-Case
The model estimated that the impact of PHiD-CV and PCV13 was broadly similar for meningitis, other IPD and all-cause pneumonia in the base-case analysis (Table 4). However, PHiD-CV was projected to have a more pronounced impact than PCV13 on AOM-related outcomes by preventing over 3600 incremental AOM-related cases. This resulted in an incremental AOM-related savings of over NZD 1 million and overall savings of NZD 0.8 million from a payer perspective. Assuming price-parity, PHiD-CV dominated PCV13.
3.2 Herd-Effect Impact
Assuming a net indirect effect of PHiD-CV and PCV13 of 30% and 32%, respectively, on IPD across all age-groups, PHiD-CV dominated PCV13. PCV13, compared to PHiD-CV, prevented 2.0, 17.8 and 0.5 cases of IPD meningitis, other IPD and meningitis sequelae, respectively (Table 4). The disproportionate impact on other IPD can be explained by the fact that the incidence in the > 65-year-old population is 24 times higher than IPD meningitis. Overall, the use of PHiD-CV would still result in 11.6 quality-adjusted life-years (QALYs) gained and cost savings of NZD 721,478 compared to PCV13 (discounted) (Table 4).
3.3 Indirect Costs Impact
The inclusion of indirect costs into the base-case analysis results in incremental (discounted) cost savings of NZD 992,510 for PHiD-CV over PCV13 (Table 4).
3.4 Sensitivity Analysis
The results of the one-way sensitivity analysis (ranges presented in Supplementary Table 3) (assuming no net indirect effect) are presented in Fig. 3. The key differentiators for PHiD-CV over PCV13, namely those relating to AOM, are the major drivers of the results.
One-way sensitivity analysis results. Red bars denote the change from the baseline ICER when the upper limit of the parameter is used in the analysis and the blue bars when the lower limit of the parameter is used. AOM acute otitis media, CI confidence interval, ICER Incremental Cost-Effectiveness Ratio, IPD invasive pneumococcal disease, GP general practitioner, NTHi non-typeable Haemophilus influenzae, PHiD-CV 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine, QALY quality-adjusted life-year
The results of the probabilistic sensitivity analysis (PSA) are presented in Fig. 4. PHiD-CV dominated PCV13 in 80.5% of the 1000 runs, while being dominated by PCV13 in 18.8% of the simulations.
Probabilistic sensitivity analysis (PSA). NZD New Zealand dollars, QALY quality-adjusted life-year. Blue markers represent individual probabilistic simulations; the red marker represents the base-case estimate; and the grey line represents a hypothetical threshold for the maximum willingness-to-pay of NZD 56,637 (2016 1xGDP per capita) for illustrative purposes (since PHARMAC does not use a cost-effectiveness threshold for decision-making)
4 Discussion
Our analysis estimated a similar impact of PHiD-CV and PCV13 against IPD and all-cause pneumonia in the base-case analysis. PHiD-CV was, however, projected to result in cost-savings from a payer-perspective of approximately NZD 0.8 million over PCV13 by preventing over 3600 incremental AOM-related cases.
For this economic assessment comparing PHiD-CV with PCV13 in NZ, we incorporated the most robust clinical trial data available and supplemented data gaps with real-world effectiveness data. Model inputs regarding VE have been validated through expert reviews and advisory boards and are based on studies of PCV7 [23, 48], PHiD-CV [40, 44] and its precursor [49]. All epidemiological and cost inputs are either direct extracts of the most recently available NZ data, or derived from the most relevant source in reference countries (usually the UK).
Since price-parity was assumed, in an effort to focus on the clinical differences between the two vaccines, along with no change in the schedule, the model outcome is highly dependent on the specifics of VE attributed to each vaccine. While the simplest expectation that differences in VE would correspond to the serotype coverage of each vaccine, the available data indicate the reality is more complex.
For the ten common serotypes, both vaccines are considered to be more or less equal, but for the three additional serotypes it is important to look at the available effectiveness data for both vaccines. There is still no conclusive evidence that PCV13 prevents IPD caused by serotype 3 [29,30,31,32,33, 35]. For 19A, full (94.7%) efficacy is assumed for PCV13 despite observed evidence of a lower protection compared to other vaccine types [29, 34, 70,71,72]. Early economic assessments assumed little or no cross-reactivity by PHiD-CV for 19A; however, recent data strongly support the basis for the inclusion of protection [37,38,39,40]. These studies were considered robust enough for the European Medicines Agency (EMA) and NZ Medicines and Medical Devices Safety Authority (Medsafe) to update the PHiD-CV summary of product characteristics (SmPC) in Europe and the product data sheet, respectively, to include protection against 19A [73, 74]. Furthermore, a recent systematic review of PCV effectiveness in children < 5 years of age in Latin America and the Caribbean concluded that both vaccines offered similar protection not just for pneumonia, but also for IPD [45].
However, substantial differences exist between these two vaccines for the prevention of AOM. The efficacy of PCV7 against AOM in the Finnish Otitis Media (FinOM) study [48] was much lower than that of the PHiD-CV precursor in the POET study [49] (7 vs. 34%), and the recent COMPAS trial demonstrated a 19% efficacy against clinical AOM for PHiD-CV [44]. While these trials are not directly comparable, PHiD-CV would be expected to have a greater impact on AOM due to its novel protein D carrier by providing the opportunity to reduce the two leading causes of OM, Spn and NTHi [75, 76]. The positive point estimate of PHiD-CV efficacy against NTHi observed in COMPAS (22%; 95% CI – 43 to 57) [44] and the significant efficacy observed with the 11-valent vaccine formulation in POET (35%; 95% CI 2–57) [49] are in stark contrast with the negative estimates observed for non-protein D containing PCVs [48, 77]. While the vaccine formulations used in the POET and COMPAS trials are different, Schuerman et al. observed that antibody responses against protein D for the 11-valent Pneumococcal Protein D (11Pn-PD) conjugate vaccine formulation were in the same range as those measured with the 10-valent PHiD-CV vaccine [78]. Given the burden of AOM in NZ, it is not surprising that this difference in vaccine effectiveness weighs heavily on the results.
The current comparison indicates that there are simultaneous cost and QALY benefits to be expected with PHiD-CV use over PCV13. There are a small number of IPD cases (and even smaller numbers of sequelae and deaths) potentially saved through PCV13 use; however, the large reductions in AOM-related events with PHiD-CV use are expected to result in costs and QALY benefits. These benefits were observed over all the scenarios tested in the one-way sensitivity analysis. These reductions in AOM are particularly relevant given the disproportionate burden of disease afflicting Maori and Pacific Island children in NZ [7, 8]. Recurrent OM can also potentially lead to hearing impairment if left untreated and has been associated with social and developmental problems [5, 6, 79] and the use of PHiD-CV over PCV13 could, potentially, help address the health equity gap in NZ.
Some limitations in the analysis proposed here need to be highlighted. Dynamic transmission models to assess the impact of vaccination against pneumococcal disease have the benefit of incorporating the indirect benefits of vaccination as well as more accurately modelling the dynamic nature of the disease [80,81,82]. The choice of a static cohort model for our analysis was driven by factors such as simplicity of modelling and its accessibility despite being non-optimal for dealing with the dynamic nature of pneumococcal diseases and the temporal impact of the vaccine in the overall population. While NZ-specific hospitalization data are available for IPD, pneumonia and AOM over the period of PCV usage [17], such observational data are prone to a variety of biases given the complex nature of pneumococcal diseases, the lack of detailed reporting and the change of PCVs every 3-year period [83]. We, thus, used data obtained from randomised trials and other robustly designed studies in a prospective static model to estimate the comparative life-time benefits of either vaccine for a single birth cohort.
Given the disproportionate burden of IPD in Maori and Pacific Islander children the use of aggregated cost and epidemiological data for the analysis, may not be able to estimate the value of either vaccine in these groups. A further detailed analysis using patient-level data and vaccine impact across various ethnic or income groups will be consequently necessary.
We estimated the herd effect in a static model using a fixed approach. There are currently not enough data available on PCVs to estimate when a steady-state is expected to be reached in a population and what the net effect could be over time. IPD serotype replacement has been observed in various countries that have implemented mass-vaccination with PCVs—the extent of which in both the vaccinated and unvaccinated vary considerably across studies [84]. Given the limitations of observational studies, it is not possible to predict accurately how much of the variance in the effect can be attributed to true differences or to surveillance artefacts. Our analysis has not taken into account this phenomenon but since we expect a similar level of serotype replacement to occur for both higher-valent vaccines, the impact of serotype replacement on the incremental effects is expected to be small.
Surveillance reports of IPD during periods of use of PHiD-CV in various countries have shown that IPD caused by 19A has not decreased in line with the protection estimated from well-designed studies [55, 85]. The exact reasons for this contrast are not exactly known, but it is important to note that the analysis of secular trends from surveillance reports may be biased by differences in the ascertainment of disease, changes in diagnostic criteria and other confounding factors. A similar phenomenon can be observed in Australia where PCV13 replaced PCV7 in the NIP in 2011. While a significant drop of 61% was seen in 19A cases in children < 5 years of age (from 7.05/100,000 in 2011 to 2.76/100,000 in 2012), 19A continues to cause IPD in this age group with approximately 30 cases annually [86]. Reports from the UK and Ireland, both of which have used PCV13 over a long period of time, have also observed similar trends [87, 88]. To account for the potential variability in the effectiveness of PHiD-CV against 19A IPD, the 95% CI estimated by Verani et al. (16.6–90.1) were used in the sensitivity analysis. Owing to the relatively low incidence of 19A in NZ, PHiD-CV still came out as the dominant option compared to PCV13.
Robust evidence on the protection conferred by PHiD-CV against NTHi AOM is sparse. However, the available evidence points to a protective effect [44, 49, 75, 76, 89]. In a study that looked at NPC and middle-ear discharge (ED) microbiology in indigenous Australian children vaccinated with PCVs, the prevalence of NTHi-infected ED was lower in PHiD-CV vaccinated children (34%) compared to PCV7 vaccinated children (61%) [89]. Concurrently, there was no substantial difference in the NPC of children vaccinated with PCV7 compared to PHiD-CV [89]. The authors hypothesised that vaccine-induced immune responses could deliver protection in the middle ear where the numbers of organisms were likely to be lower, without eliminating NPC [89]. A follow-up study in the same population following a switch from PHiD-CV to PCV13 showed that while children vaccinated with PCV13 had significantly lower carriage of combined Spn and NTHi, there was no change in disease severity or OM prevalence. In non-mixed PCV schedules, children receiving PCV13 had lower NTHi carriage as well. An analysis of ED swabs, however, showed a higher prevalence of middle ear co-infection with NTHi and Spn in children vaccinated with PCV13 compared to PHiD-CV [90]. Further studies are required to robustly estimate the effect of PHiD-CV on NTHi AOM.
5 Conclusion
In our analysis both vaccines are predicted to provide a broadly comparable impact on IPD-related diseases and pneumonia. Importantly, due to the additional benefits possible through a broader impact on AOM, PHiD-CV is estimated to potentially provide additional cost offsets of approximately NZD 0.8 million over the lifetime of a single birth cohort at equal vaccine acquisition costs. The results of this analysis have been reflected in PHARMAC’s decision to replace PCV13 with PHiD-CV in 2017 [11].
Direct comparison of the antigenic content of PHiD-CV and PCV13 should not be used on its own to differentiate the potential benefits of the two vaccines. While PCV13 contains antigens for an additional three Spn serotypes, the incremental value of serotype 3 is unclear for PCV13 and significant cross-reactive protection for PHiD-CV against 6A [40] and 19A [37,38,39,40] has been demonstrated.
OM is a significant driver of healthcare costs, and is responsible for approximately 70% of the net present lifetime cost of PCV-preventable diseases in children [7, 21, 66]. PHiD-CV has the potential to provide protection against AOM caused by NTHi [44, 49]. A vaccine with the potential for dual protection against both Spn and NTHi could offer substantially greater benefits than a vaccine covering Spn only [91]. Importantly, vaccination against key otopathogens is not only expected to reduce the burden of OM [92], but preventing OM in the first year of life is likely to have the greatest impact as these early episodes are more likely to lead to recurrence [93].
References
World Health Organisation. Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008. In: http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/. Accessed 19 Jan 2016.
Ministry of Health. Immunisation Handbook 2014. In: http://www.health.govt.nz/publication/immunisation-handbook-2014. Accessed 30 Oct 2015.
Institute of Environmental Science and Research (ESR). Invasive pneumococcal disease in New Zealand, 2014. In: https://surv.esr.cri.nz/PDF_surveillance/IPD/2014/2014IPDAnnualReport.pdf. Accessed 29 Jun 2016.
Mills N, Best EJ, Murdoch D, et al. What is behind the ear drum? The microbiology of otitis media and the nasopharyngeal flora in children in the era of pneumococcal vaccination. J Paediatr Child Health. 2015;51(3):300–6.
Greenberg D, Bilenko N, Liss Z, et al. The burden of acute otitis media on the patient and the family. Eur J Pediatr. 2003;162(9):576–81.
O’Connor TE, Perry CF, Lannigan FJ. Complications of otitis media in Indigenous and non-Indigenous children. Med J Aust. 2009;191(9 Suppl):S60–4.
Milne RJ, Vander Hoorn S. Burden and cost of hospital admissions for vaccine-preventable paediatric pneumococcal disease and non-typable Haemophilus influenzae otitis media in New Zealand. Appl Health Econ Health Policy. 2010;8(5):281–300.
McCallum J, Craig L, Whittaker I, Baxter J. Ethnic differences in acute hospitalisations for otitis media and elective hospitalisations for ventilation tubes in New Zealand children aged 0-14 years. N Z Med J. 2015;128(1416):10–20.
Telfar Barnard L, Baker M, Pierse N, Zhang J. The impact of respiratory disease in New Zealand: 2014 update. In: https://www.asthmafoundation.org.nz/research/the-impact-of-respiratory-disease-in-new-zealand-2014-update. Accessed 14 Sep 2016.
PHARMAC. Changes to the National Immunisation Schedule—2013. In: https://www.pharmac.health.nz/assets/notification-2013-12-17-national-immunisation-schedule.pdf. Accessed 1 Dec 2015.
PHARMAC. Changes to the National Immunisation Schedule—2016. In: https://www.pharmac.govt.nz/news/notification-2016-07-28-immunisation-schedule. Accessed 1 Feb 2018.
Knerer G, Ismaila A, Pearce D. Health and economic impact of PHiD-CV in Canada and the UK: a Markov modelling exercise. J Med Econ. 2012;15(1):61–76.
PHARMAC. Prescription for Pharmacoeconomic Analysis v2.2. In: https://www.pharmac.govt.nz/assets/pfpa-2-2.pdf. Accessed 14 Dec 2015.
Statistics New Zealand. Births and Deaths (Year ended Dec 2014). In: http://www.stats.govt.nz/browse_for_stats/population/births/BirthsAndDeaths_HOTPYeDec14.aspx. Accessed 18 Sep 2015.
Statistics New Zealand. Age-specific death rates by sex and age, 2014. In: http://www.stats.govt.nz/infoshare/. Accessed 18 Sep 2015.
Morrow A, De WP, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol. 2007;18(2):121–7.
Ministry of Health. Publicly funded hospital discharges—1 July 2012 to 30 June 2013 (Table 5). In: http://www.health.govt.nz/system/files/documents/publications/pubdischargesprocedures2012-13_pivotfinal_0.xlsb. Accessed 23 Sep 2015.
Ministry of Health. Mortality 2012 online tables. In: http://www.health.govt.nz/system/files/documents/publications/mortality_2012_online_tables_-_finalupdated28aug15.xlsx. Accessed 23 Sep 2015.
Delgleize E, Leeuwenkamp O, Theodorou E, Van de Velde N. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open. 2016;6(11):e010776.
Park H, Adeyemi AO, Rascati KL. Direct Medical Costs and Utilization of Health Care Services to Treat Pneumonia in the United States: An Analysis of the 2007-2011 Medical Expenditure Panel Survey. Clin Ther. 2015;37(7):1466–76.e1.
Gribben B, Salkeld LJ, Hoare S, Jones HF. The incidence of acute otitis media in New Zealand children under five years of age in the primary care setting. J Prim Health Care. 2012;4(3):205–12.
Melegaro A, Edmunds WJ, Pebody R, Miller E, George R. The current burden of pneumococcal disease in England and Wales. J Infect. 2006;52(1):37–48.
Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–502.
Madhi SA, Adrian P, Kuwanda L, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2007;25(13):2451–7.
Le Polain De Waroux O, Flasche S, Prieto-Merino D, Goldblatt D, Edmunds WJ. The efficacy and duration of protection of pneumococcal conjugate vaccines against nasopharyngeal carriage: a meta-regression model. Pediatr Infect Dis J. 2015;34(8):858–64.
Kieninger DM, Kueper K, Steul K, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010;28(25):4192–203.
Vesikari T, Wysocki J, Chevallier B, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J. 2009;28(4 Suppl):S66–76.
Yeh SH, Gurtman A, Hurley DC, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics. 2010;126(3):e493–505.
Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46.
Demczuk WH, Martin I, Griffith A, et al. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010–2012. Can J Microbiol. 2013;59(12):778–88.
Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59(8):1066–73.
Kaplan SL, Barson WJ, Lin PL, et al. Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2013;32(3):203–7.
Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–9.
Moore MR, Link-Gelles R, Schaffner W, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016;4(5):399–406.
Steens A, Bergsaker MA, Aaberge IS, Ronning K, Vestrheim DF. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine. 2013;31(52):6232–8.
Poolman J, Frasch C, Nurkka A, et al. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin Vaccine Immunol. 2011;18(2):327–36.
Domingues CM, Verani JR, Montenegro Renoiner EI, et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med. 2014;2(6):464–71.
Verani JR, Domingues CM, Moraes JC. Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Indirect cohort analysis of 10-valent pneumococcal conjugate vaccine effectiveness against vaccine-type and vaccine-related invasive pneumococcal disease. Vaccine. 2015;33(46):6145–8.
Deceuninck G, De Wals P, Boulianne N, De Serres G. Effectiveness of pneumococcal conjugate vaccine using a 2 + 1 infant schedule in Quebec. Canada. Pediatr Infect Dis J. 2010;29(6):546–9.
Jokinen J, Rinta-Kokko H, Siira L, et al. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children–a population-based study. PLoS ONE. 2015;10(3):e0120290.
Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–5.
Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25(9):779–81.
Kilpi T, Palmu AA, Puumalainen T, et al. Effectiveness of the 10-valent pneumococcal Haemophilus Influenzae protein D conjugate vaccine (PHID-CV10) against hospital-diagnosed pneumonia in infants- FinIP trial. Abstract presented at The 31st Annual Meeting of the European Society for Pediatric Infectious Diseases (ESPID)—May 28-June 1, 2013, Milan, Italy. In: http://w3.kenes-group.com/apps/espid2013/abstracts/pdf/134.pdf. Accessed 1 Dec 2015.
Tregnaghi MW, Saez-Llorens X, Lopez P, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 2014;11(6):e1001657.
de Oliveira LH, Camacho LA, Coutinho ES, et al. Impact and effectiveness of 10 and 13-valent pneumococcal conjugate vaccines on hospitalization and mortality in children aged less than 5 years in latin american countries: a systematic review. PLoS ONE. 2016;11(12):e0166736. https://doi.org/10.1371/journal.pone.0166736.
Leibovitz E, Greenberg D. Acute otitis media in children: current epidemiology, microbiology, clinical manifestations, and treatment. Chang Gung Med J. 2004;27(7):475–88.
Hausdorff WP, Yothers G, Dagan R, et al. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr Infect Dis J. 2002;21(11):1008–16.
Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–9.
Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367(9512):740–8.
Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19(3):187–95.
Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004;23(12):1142–52.
Davis SM, Deloria-Knoll M, Kassa HT, O’Brien KL. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine. 2013;32(1):133–45.
Jokinen J, Kilpi TM, Kaijalainen T, et al. Indirect effectiveness of pneumococcal haemophilus influenzae protein-D conjugate vaccine (PHiD-CV10) against oropharyngeal and nasopharyngeal carriage—FinIP indirect carriage study. Abstract ISPPD-0395 presented at the 9th International Symposium on Pneumococci & Pneumococcal Diseases—9th-13th March, 2014, Hyderabad, India. In: https://pneumonia.org.au/public/journals/22/ABSTRACTBOOKMASTERforwebupdated20-3-14.pdf.
Institute of Environmental Science & Research. Invasive Pneumococcal Disease Reports. In: https://surv.esr.cri.nz/surveillance/IPD.php. Accessed 9 Mar 2015.
National Institute for Health and Welfare. Incidence of invasive pneumococcal disease in Finland. In: https://www.thl.fi/en/web/thlfi-en/research-and-expertwork/projects-and-programmes/monitoring-the-population-effectiveness-of-pneumococcal-conjugate-vaccination-in-the-finnish-national-vaccination-programme/incidence-of-invasive-pneumococcal-disease-in-finland. Accessed 9 Mar 2015.
Public Health Agency of Canada. National Laboratory Surveillance of Invasive Streptococcal Disease in Canada—Annual Summary 2013. In: http://www.healthycanadians.gc.ca/publications/drugs-products-medicaments-produits/2013-streptococcus/index-eng.php. Accessed 9 Mar 2015.
Centers for Disease Control and Prevention (CDC). Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54(36):893–7.
Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. Springer, Dordrecht, Netherlands, 2014.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14.
Bennett JE, Sumner W, Downs SM, Jaffe DM. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154(1):43–8.
Vold Pepper P, Owens DK. Cost-effectiveness of the pneumococcal vaccine in the United States Navy and Marine Corps. Clin Infect Dis. 2000;30(1):157–64.
Oh PI, Maerov P, Pritchard D, et al. A cost-utility analysis of second-line antibiotics in the treatment of acute otitis media in children. Clin Ther. 1996;18(1):160–82.
Cheng AK, Niparklo JK. Cost-utility of the cochlear implant in adults: a meta-analysis. Arch Otolaryngol Head Neck Surg. 1999;125(11):1214–8.
PHARMAC. Cost Resource Manual Version 2.2 (October 2015). In: https://www.pharmac.health.nz/assets/pfpa-v2-2-cost-resource-manual.pdf. Accessed 14 Dec 2015.
Ministry of Health. Weighted Inlier Equivalent Separations (2015/16). In: http://www.health.govt.nz/nz-health-statistics/data-references/weighted-inlier-equivalent-separations. Accessed 14 Dec 2015.
Milne RJ, Vander Hoorn S. An economic evaluation of replacement of 7-valent pneumococcal vaccine with either a 10-valent or a 13-valent pneumococcal vaccine on the New Zealand childhood immunisation schedule—updated Report to the New Zealand Ministry of Health. GlaxoSmithKline NZ Ltd; 2009.
Nunes MC, Madhi SA. Review on the immunogenicity and safety of PCV-13 in infants and toddlers. Expert Rev Vaccines. 2011;10(7):951–80.
Silfverdal SA, Coremans V, François N, Borys D, Cleerbout J. Safety profile of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Expert Rev Vaccines. 2017;16(2):109–21.
Statistics New Zealand. Income for all people by labour force status, sex, and age groups (2015). In: http://nzdotstat.stats.govt.nz/wbos/Index.aspx?DataSetCode=TABLECODE7458#. Accessed 20 Sep 2016.
Deceuninck G, De Serres G, Boulianne N, Lefebvre B, De Wals P. Effectiveness of three pneumococcal conjugate vaccines to prevent invasive pneumococcal disease in Quebec, Canada. Vaccine. 2015;33(23):2684–9.
Su WJ, Lo HY, Chang CH, et al. Effectiveness of pneumococcal conjugate vaccines of different valences against invasive pneumococcal disease among children in taiwan: a nationwide study. Pediatr Infect Dis J. 2016;35(4):e124–33.
Weinberger R, van der Linden M, Imöhl M, von Kries R. Vaccine effectiveness of PCV13 in a 3 + 1 vaccination schedule. Vaccine. 2016;34(18):2062–5.
European Medicines Agency. Summary of Product Characteristics—Synflorix. In: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000973/WC500054346.pdf. Accessed 1 Dec 2015.
New Zealand Medicines and Medical Devices Safety Authority (Medsafe). Data Sheet—Synflorix. In: http://www.medsafe.govt.nz/profs/Datasheet/s/synflorixinj.pdf. Accessed 25 Jan 2017.
Croxtall JD, Keating GM. Pneumococcal polysaccharide protein D-conjugate vaccine (Synflorix; PHiD-CV). Paediatr Drugs. 2009;11(5):349–57.
Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: synflorix. Expert Rev Vaccines. 2009;8(11):1479–500.
Kilpi T, Ahman H, Jokinen J, et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin Infect Dis. 2003;37(9):1155–64.
Schuerman L, Borys D, Hoet B, Forsgren A, Prymula R. Prevention of otitis media: now a reality? Vaccine. 2009;27(42):5748–54.
Klein JO. The burden of otitis media. Vaccine. 2000;19(Suppl 1):S2–8.
De Cao E, Melegaro A, Klok R, Postma M. Optimising assessments of the epidemiological impact in The Netherlands of paediatric immunisation with 13-valent pneumococcal conjugate vaccine using dynamic transmission modelling. PLoS One. 2014;9(4):e89415.
Melegaro A, Choi YH, Georges R, et al. Dynamic models of pneumococcal carriage and the impact of the heptavalent pneumococcal conjugate vaccine on invasive pneumococcal disease. BMC Infect Dis. 2010;10:90. https://doi.org/10.1186/1471-2334-10-90.
van Hoek AJ, Choi YH, Trotter C, Miller E, Jit M. The cost-effectiveness of a 13-valent pneumococcal conjugate vaccination for infants in England. Vaccine. 2012;30(50):7205–13.
Bruhn CA, Hetterich S, Schuck-Paim C, et al. Estimating the population-level impact of vaccines using synthetic controls. Proc Natl Acad Sci USA. 2017;114(7):1524–9.
Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–73.
Andrade AL, Minamisava R, Policena G, et al. Evaluating the impact of PCV-10 on invasive pneumococcal disease in Brazil: a time-series analysis. Hum Vacc Immunother. 2016;12(2):285–92.
National Notifiable Diseases Surveillance System (Department of Health—Australia). Pneumococcal disease (invasive) Public dataset. In: http://www9.health.gov.au/cda/source/display_doc.cfm?DocID=7. Accessed 10 Feb 2017.
Collins S, Sheppard C, Litt D, et al. Trends in invasive pneumococcal disease over time: England and Wales 2000/01 to 2014/15. Abstract 509 presented at the 10th International Symposium on Pneumococci & Pneumococcal Diseases—26th-30th June, 2016, Glasgow, Scotland. In: http://www.isppd2016.kenes.com/scientific-program-%282%29/scientific-program-2#.WJMBhvkrLIV. Accessed 20 Sept 2016.
Corcoran M, Vickers I, Fitzgerald M, et al. The persistence of serotype 19A—despite the introduction of PCV13 vaccine. Abstract 516 presented at the 10th International Symposium on Pneumococci & Pneumococcal Diseases—26th-30th June, 2016, Glasgow, Scotland. In: http://www.isppd2016.kenes.com/scientific-program-%282%29/scientific-program-2#.WJMBhvkrLIV. Accessed 20 Sept 2016.
Leach AJ, Wigger C, Hare K, et al. Reduced middle ear infection with non-typeable Haemophilus influenzae, but not Streptococcus pneumoniae, after transition to 10-valent pneumococcal non-typeable H. influenzae protein D conjugate vaccine. BMC Pediatr. 2015;15:162.
Leach AJ, Wigger C, Beissbarth J, et al. General health, otitis media, nasopharyngeal carriage and middle ear microbiology in Northern Territory Aboriginal children vaccinated during consecutive periods of 10-valent or 13-valent pneumococcal conjugate vaccines. Int J Pediatr Otorhinolaryngol. 2016;86:224–32.
Stubbs E, Hare K, Wilson C, Morris P, Leach AJ. Streptococcus pneumoniae and noncapsular Haemophilus influenzae nasal carriage and hand contamination in children: a comparison of two populations at risk of otitis media. Pediatr Infect Dis J. 2005;24(5):423–8.
Grevers G. Challenges in reducing the burden of otitis media disease: an ENT perspective on improving management and prospects for prevention. Int J Pediatr Otorhinolaryngol. 2010;74(6):572–7.
Murphy TF, Bakaletz LO, Kyd JM, Watson B, Klein DL. Vaccines for otitis media: proposals for overcoming obstacles to progress. Vaccine. 2005;23(21):2696–702.
Acknowledgements
The authors would like to acknowledge Richard Milne PhD for his contribution to the study and his critical review of the manuscript. The authors also would like to thank Business and Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Nathalie Arts coordinated manuscript development and editorial support.
Data Availability Statement
Data that are detailed in the methods or in the supplementary material can be found in the list of references contained in the manuscript.
Author information
Authors and Affiliations
Contributions
All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. BM, LT and XHZ conceived and designed the study. The data were acquired and analysed by all authors. All authors participated in the development of this manuscript and gave final approval before submission.
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of the GSK group of companies. BM and XHZ hold shares in the GSK group of companies. GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and agreed with the submission of the publication.
Trademark section
Synflorix is a trade mark owned by or licensed to the GSK group of companies. Prevenar is a trade mark of Wyeth LLC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Varghese, L., Talbot, L., Govender, A. et al. A Cost-Effectiveness Analysis of the 10-Valent Pneumococcal Non-Typeable Haemophilus influenzae Protein D Conjugate Vaccine (PHiD-CV) Compared to the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) for Universal Mass Vaccination Implementation in New Zealand. Appl Health Econ Health Policy 16, 331–345 (2018). https://doi.org/10.1007/s40258-018-0387-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-018-0387-5