Abstract
Several classes of new oral therapy are in use or in development for the treatment of psoriasis. Despite the high efficacy of biologics, new oral therapies remain important as patients generally prefer this mode of administration and they offer an alternative risk–benefit profile. In this review, we discuss the novel modes of action of these drugs, including modulation of cellular pathways involving diverse targets such as Janus kinase, phosphodiesterase 4, sphingosine 1-phosphate, A3 adenosine receptor and rho-associated kinase 2. We review the available evidence around licensed drugs (apremilast) and drugs that are advanced (tofacitinib) or early (ponesimod, baricitinib, peficitinib, INCB039110, CF101, KD025) in the development pipeline. The key limitations of these oral therapies are their modest efficacy profile (apremilast, ponesimod) and the limitations of their safety profile (tofacitinib, ponesimod), while the evidence for the early pipeline drugs are at phase II level only. Potential niches of current unmet needs include apremilast for patients with concomitant psoriatic arthritis, as combination treatments with biologic therapies, and/or for patients in whom multiple biologic therapies have failed due to immunogenicity and secondary inefficacy. The present knowledge gap regarding these novel drugs includes the need for longer clinical trials or observational studies to evaluate safety, and randomised phase III trials for the early pipeline drugs. We conclude that further research and data are necessary to conclusively establish the role of these agents in the current psoriasis treatment paradigm.
Similar content being viewed by others
References
Edmundson WF, Guy WB. Treatment of psoriasis with folic acid antagonists. AMA Arch Dermatol. 1958;78(2):200–3.
Warren RB, Chalmers RJG, Griffiths CEM, Menter A. Methotrexate for psoriasis in the era of biological therapy. Clin Exp Dermatol. 2008;33(5):551–4.
Griffiths CEM, Barker JNWN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–71.
Yiu ZZ, Griffiths CE. Interleukin 17-A inhibition in the treatment of psoriasis. Expert Rev Clin Immunol. 2016;12(1):1–4.
Warren RB, Smith CH, Yiu ZZN, Ashcroft DM, Barker JNWN, Burden AD, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135(11):2632–40.
D’Souza LS, Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2015;72(4):589–98.
Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Fu B, Ustianowski AP, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatol Oxf Engl. 2011;50(1):124–31.
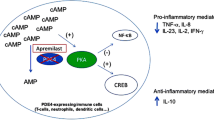
Schafer PH, Parton A, Capone L, Cedzik D, Brady H, Evans JF, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–29.
Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159(4):842–55.
Gottlieb AB, Strober B, Krueger JG, Rohane P, Zeldis JB, Hu CC, et al. An open-label, single-arm pilot study in patients with severe plaque-type psoriasis treated with an oral anti-inflammatory agent, apremilast. Curr Med Res Opin. 2008;24(5):1529–38.
Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–99.
Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–6.
Mease P, Adebajo A, Gladman D, Gomez-Reino J, Hall S, Kavanaugh A, et al. THU0432 long-term (104-week) safety profile of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: pooled safety analysis of three phase 3, randomized, controlled trials. Ann Rheum Dis. 2015;74(Suppl 2):355–6.
Reich K, Sobell J, Day RM, Stevens RM, Shah K. Change in weight with apremilast, an oral phosphodiesterase 4 inhibitor: pooled analysis of the ESTEEM 1 and ESTEEM 2 trials. J Am Acad Dermatol. 2015;72(5):AB227.
Mease PJ. Apremilast: a phosphodiesterase 4 inhibitor for the treatment of psoriatic arthritis. Rheumatol Ther. 2014;1(1):1–20.
Burden AD, Warren RB, Kleyn CE, McElhone K, Smith CH, Reynolds NJ, et al. The British Association of Dermatologists’ Biologic Interventions Register (BADBIR): design, methodology and objectives. Br J Dermatol. 2012;166(3):545–54.
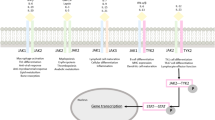
Chang BY, Zhao F, He X, Ren H, Braselmann S, Taylor V, et al. JAK3 inhibition significantly attenuates psoriasiform skin inflammation in CD18 mutant PL/J mice. J Immunol Baltim Md 1950. 2009;183(3):2183–92.
Andrés RM, Hald A, Johansen C, Kragballe K, Iversen L. Studies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activity. Exp Dermatol. 2013;22(5):323–8.
Bachelez H, van de Kerkhof PCM, Strohal R, Kubanov A, Valenzuela F, Lee J-H, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–61.
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–86.
Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167(3):668–77.
Kaur K, Kalra S, Kaushal S. Systematic review of tofacitinib: a new drug for the management of rheumatoid arthritis. Clin Ther. 2014;36(7):1074–86.
D’Ambrosio D, Steinmann J, Brossard P, Dingemanse J. Differential effects of ponesimod, a selective S1P1 receptor modulator, on blood-circulating human T cell subpopulations. Immunopharmacol Immunotoxicol. 2015;37(1):103–9.
Piali L, Froidevaux S, Hess P, Nayler O, Bolli MH, Schlosser E, et al. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J Pharmacol Exp Ther. 2011;337(2):547–56.
Vaclavkova A, Chimenti S, Arenberger P, Holló P, Sator P-G, Burcklen M, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Lond Engl. 2014;384(9959):2036–45.
Ryan C, Menter A. Ponesimod: a future oral therapy for psoriasis? Lancet. 2014;384(9959):2006–8.
Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167(5):1035–47.
Roviezzo F, Di Lorenzo A, Bucci M, Brancaleone V, Vellecco V, De Nardo M, et al. Sphingosine-1-phosphate/sphingosine kinase pathway is involved in mouse airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2007;36(6):757–62.
Yiu ZZN, Warren RB, Mrowietz U, Griffiths CEM. Safety of conventional systemic therapies for psoriasis on reproductive potential and outcomes. J Dermatol Treat. 2015;30:1–6.
Yiu ZZN, Griffiths CEM, Warren RB. Safety of biological therapies for psoriasis: effects on reproductive potential and outcomes in male and female patients. Br J Dermatol. 2014;171(3):485–91.
Menter A, Disch D, Clemens J, Janes J, Papp K, Macias W. A phase 2b trial of baricitinib, an oral JAK inhibitor, in patients with moderate to severe psoriasis. J Am Acad Dermatol. 2014;70(5):AB162.
Papp K, Pariser D, Catlin M, Wierz G, Ball G, Akinlade B, et al. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2015;173(3):767–76.
Bissonnette R, Luchi M, Fidelus-Gort R, Jackson S, Zhang H, Flores R, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of the safety and efficacy of INCB039110, an oral janus kinase 1 inhibitor, in patients with stable, chronic plaque psoriasis. J Dermatol Treat. 2016;14:1–7.
David M, Akerman L, Ziv M, Kadurina M, Gospodinov D, Pavlotsky F, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol JEADV. 2012;26(3):361–7.
Müller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta. 2011;1808(5):1290–308.
Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, Chen W, Scher JU, Mo R, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci USA. 2014;111(47):16814–9.
Psoriasis (plaque, moderate to severe)—apremilast [ID679] | Guidance and guidelines | NICE [Internet]. [cited 2015 Aug 5]. Available from https://www.nice.org.uk/guidance/indevelopment/gid-tag469/documents.
Scottish Medicines Consortium apremilast (Otezla) CPP [Internet]. [cited 2015 Aug 5]. Available from https://www.scottishmedicines.org.uk/SMC_Advice/Advice/1052_15_apremilast_Otezla_plaque_psoriasis/apremilast_Otezla_plaque_psoriasis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
ZZNY has no conflicts of interest to declare. RBW has acted as a consultant and/or speaker and/or received research grants for Abbvie, Amgen, Celgene, Boehringer, Eli Lilly, Pfizer, Leo, Novartis, Xenoport and Janssen.
Funding
No financial assistance was received for the preparation of the manuscript.
Rights and permissions
About this article
Cite this article
Yiu, Z.Z.N., Warren, R.B. Novel Oral Therapies for Psoriasis and Psoriatic Arthritis. Am J Clin Dermatol 17, 191–200 (2016). https://doi.org/10.1007/s40257-016-0179-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-016-0179-3