Abstract
Background
Information is lacking on long-term management of patients with acute coronary syndrome (ACS) and chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2).
Objectives
Our objectives were to describe antithrombotic management patterns and outcomes in patients with ACS with varying renal function from the EPICOR (long-tErm follow-uP of antithrombotic management patterns In acute CORonary syndrome patients; NCT01171404) and EPICOR Asia (NCT01361386) studies.
Methods
EPICOR and EPICOR Asia were prospective observational studies of patients who survived hospitalization for ACS and were enrolled at discharge in 28 countries across Europe, Latin America, and Asia. The studies were conducted from 2010 to 2013 and from 2011 to 2014, respectively. This analysis evaluated patient characteristics and oral antithrombotic management patterns and outcomes up to 2 years post-discharge according to admission eGFR: ≥ 90, 60–89, 30–59, or < 30 mL/min/1.73 m2.
Results
Among 22,380 patients with available data, eGFR < 60 mL/min/1.73 m2 was observed in 16.7%. Patients with poorer renal function were older, were at greater cardiovascular risk, and had more prior cardiovascular disease and bleeding. Patients with CKD underwent fewer cardiovascular interventions and had more in-hospital cardiovascular and bleeding events. Dual antiplatelet therapy was less likely at discharge in patients with eGFR < 30 (82.3%) than in those with ≥ 90 (91.3%) mL/min/1.73 m2 and declined more sharply during follow-up in patients with low eGFR (p < 0.0001). An adjusted proportional hazards model showed that patients with lower eGFR levels had a higher risk of cardiovascular events and bleeding.
Conclusions
The presence of CKD in patients with ACS was associated with less aggressive cardiovascular management and an increased risk of cardiovascular events.
Similar content being viewed by others
Data from EPICOR and EPICOR Asia, twin international observational studies with a 2-year follow-up period, showed that chronic kidney disease (estimated glomerular filtration rate < 60 mL/min/1.73 m2) was associated with less aggressive management and an increased risk of cardiovascular events and bleeding, across all three types of acute coronary syndromes (ACS) (ST-segment elevation myocardial infarction [STEMI], non-STEMI, and unstable angina). |
Patients with ACS and renal dysfunction require careful assessment and tailored short- and long-term antithrombotic management to reduce adverse clinical event rates. |
1 Introduction
Chronic kidney disease (CKD) is associated with an increased risk of cardiovascular disease (CVD), and patients with CKD presenting with an acute coronary syndrome (ACS), particularly those with more severe renal dysfunction, have higher rates of mortality, complications, and bleeding events [1,2,3,4,5,6]. According to data from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network (ACTION) registry in the USA, CKD (defined as estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) is present in approximately 43% of patients presenting with non-ST-segment elevation (NSTE)-ACS (including NSTE myocardial infarction [NSTEMI] and unstable angina) and 31% of those with ST-segment elevation myocardial infarction (STEMI) [4].
Given the poorer prognosis for those with CKD, and the potential need for dose adjustment of some antithrombotic agents, guidelines recommend that eGFR be evaluated as soon as possible in patients presenting with ACS [7, 8]. However, the same guidelines offer little in the way of treatment guidance for patients with CKD because of the paucity of solid evidence in this regard as patients with severe renal dysfunction have been systematically excluded from many randomized clinical trials [9, 10]. There is evidence indicating that outcomes are better in patients with CKD who undergo early revascularization [11,12,13] and receive prolonged dual antiplatelet therapy (DAPT) during follow-up [14], but recent studies have suggested that they are largely undertreated compared with patients without CKD [4, 15,16,17,18]. Furthermore, there is minimal information on the effects of antithrombotic management patterns (AMPs) on long-term outcomes beyond 1 year from the index event [19].
EPICOR (long-tErm follow uP of antithrombotic management patterns In acute CORonary syndrome patients, NCT01171404) and EPICOR Asia (NCT01361386) were twin observational studies of patients surviving hospitalization for ACS who were followed-up for 2 years. The present analysis describes the association between long-term use of oral antiplatelet agents and clinical outcomes in patients with ACS with different degrees of renal function.
2 Materials and Methods
2.1 Patients
The EPICOR and EPICOR Asia study designs and baseline patient characteristics have been published previously [20, 21]. Both were multinational, observational, prospective cohort studies with 2-year follow-up periods [19]. EPICOR enrolled patients between September 2010 and March 2011 from 555 centers in 20 countries across Europe and Latin America, whereas EPICOR Asia enrolled patients between June 2011 and May 2012 from 218 centers in China, Hong Kong, India, Malaysia, Singapore, South Korea, Thailand, and Vietnam.
Patients who survived hospitalization for an ACS (STEMI, NSTEMI, or unstable angina) were eligible for inclusion if they were aged at least 18 years and were hospitalized within 24 h (EPICOR) or 48 h (EPICOR Asia) of symptom onset. For the present analysis, patients were also required to have a known level of renal function, defined by eGFR.
All patients were enrolled at discharge from hospital, which could be the original center to which they were admitted or another participating hospital to which they were transferred. Patients transferred to a non-participating hospital (and not transferred back again) were therefore excluded from the study. Other key exclusion criteria were “secondary” ACS (that is, precipitated by or occurring as a complication of surgery, trauma, percutaneous coronary intervention [PCI], or other reasons), any serious or severe comorbidities considered likely to limit life expectancy to less than 6 months, and prior enrollment in EPICOR or EPICOR Asia.
2.2 Objectives
The primary objective of EPICOR and EPICOR Asia was to describe acute and long-term AMPs in ACS survivors in a wide range of clinical settings and countries [20, 21]. The objectives of the present analysis were to describe patient characteristics, oral AMPs, and clinical outcomes over the 2-year follow-up period in patients with one of four different categories of renal function at hospital admission (baseline), defined as eGFR ≥ 90, 60–89, 30–59, or < 30 mL/min/1.73 m2. eGFR was calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine formula [22]:
where κ is 0.7 for females and 0.9 for males, α is − 0.329 for females and − 0.411 for males, and sCr is serum creatinine.
In this report, CKD is defined as patients with eGFR< 60 mL/min/1.73 m2.
2.3 Baseline and In-Hospital Variables
Collected variables included baseline demographic data, socio-economic factors, medical history (including prior CVD and non-CVD), major bleeding events within 6 months prior to the index event, medications, procedures (PCI and coronary artery bypass graft [CABG]), laboratory test results, and EuroQol 5-Dimensions (EQ-5D) simple score for quality of life. Data on medication, procedures, and outcomes (cardiovascular and bleeding events) during initial hospitalization were also recorded, as were discharge medications (including number and type of oral antiplatelet agents, anticoagulants, and other cardiovascular medications).
2.4 Outcomes
Clinical events analyzed during follow-up were death; nonfatal myocardial infarction (MI); nonfatal stroke; composite of death, MI, or stroke; first major bleeding; any bleeding; unstable angina; any coronary revascularization (PCI or CABG); and heart failure.
2.5 Statistical Analysis
Patient characteristics, AMPs, and clinical outcomes are summarized by eGFR category, showing the number and percentage of patients or mean and standard deviation for each variable, with p values from a chi-squared test for categorical variables or one-way analysis of variance for continuous variables used to assess differences between them; eGFR group was treated as a categorical variable for the purposes of these comparisons. Cox proportional hazards modeling was used to examine the association between clinical outcomes (composite cardiovascular endpoint, death, MI, ischemic stroke, bleeding, and major bleeding) and the degree of renal function (eGFR 60–89, 30–59, and < 30 with eGFR ≥ 90 mL/min/1.73 m2), estimating hazard ratios (HRs) and their 95% confidence interval (CI) for each outcome. To obtain adjusted estimates of these associations, the Cox model was fitted containing terms for age (per 10 years), sex, final diagnosis of index event, EQ-5D overall health status at discharge, in-hospital cardiac events, previous chronic obstructive pulmonary disease, previous peripheral vascular disease, prior PCI or CABG, admission glucose (< 160/≥ 160 mg/dL; cardiovascular events only), admission hemoglobin (< 13/≥ 13 g/dL), and diuretics at discharge, for subjects with data available. All p values are two-sided, with values < 0.05 considered statistically significant. All statistical analyses were performed using SAS version 9.3 or later.
3 Results
3.1 Patients
Of 23,490 patients enrolled in EPICOR (n = 10,568) and EPICOR Asia (n = 12,922), 22,380 had eGFR data available and were included in the present analysis. Baseline patient demographic and clinical characteristics are summarized by eGFR category in Table 1 and in eTable 1 in the electronic supplementary material (ESM). The majority of patients had eGFR ≥ 60 mL/min/1.73 m2 (41.9% eGFR ≥ 90 and 41.4% eGFR 60–89), whereas 16.7% had lower levels of renal function (14.5% eGFR 30–59 and 2.2% eGFR < 30). Patients with poorer renal function were older, with a lower level of education, and the percentage of male patients declined along with eGFR status. There were also differences across the country/region groups, with rates of eGFR < 30 mL/min/1.73 m2 ranging from 1.4% in China and Eastern Europe to 5.6% in Thailand, Vietnam, and Malaysia. Those same countries/regions also had the highest and lowest rates, respectively, of eGFR ≥ 90 mL/min/1.73 m2. Lower renal function was more often associated with a higher cardiovascular risk profile (with the single exception of smoking) and a greater likelihood of prior CVD, anemia, bleeding, Killip class > I, lower hemoglobin, and higher blood glucose levels. In the lowest eGFR group, more patients had NSTEMI than STEMI (46.8 vs. 37.0%). Overall, 1568 of 11,281 (12.9%) patients with STEMI and 1438 of 6285 (22.9%) patients with NSTEMI had CKD (eGFR < 60 mL/min/1.73 m2).
Mean left ventricular ejection fraction (LVEF) decreased significantly across the four renal function groups, with an increasing proportion of patients with LVEF < 30% (increasing from 1.6% in the highest renal function group to 8.0% in the lowest) (Table 1). Patients with eGFR < 60 mL/min/1.73 m2 were also more likely to have multiple diseased vessels on coronary angiography, e.g., 22.9% of patients with eGFR ≥ 90 and 40.5% with eGFR < 30 mL/min/1.73 m2 had three diseased vessels and were more likely to have left main coronary artery disease (11.5 and 18.8%, respectively).
3.2 Management and Outcomes
Despite presenting a higher risk, patients with CKD were less likely to undergo coronary angiography, PCI, or CABG during initial hospitalization and more likely to experience in-hospital cardiovascular events and bleeding (Table 2). Similar patterns of less aggressive management and increased event rates with lower eGFR were observed for patients in each ACS diagnostic category (eTable 2 in the ESM). Patients with poorer renal function were also significantly less likely to be discharged on DAPT (aspirin plus a P2Y12 inhibitor), ranging from 91.3% of patients with eGFR ≥ 90 mL/min/1.73 m2 to 82.3% of those with eGFR < 30 (p < 0.0001; Table 3). Patients with eGFR < 30 were also less likely to receive angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers. Similarly, patients with CKD were less frequently prescribed β-blockers, but more likely to receive anticoagulants, calcium channel blockers, diuretics, aldosterone inhibitors, and nitrates, whereas statin use was similar across the renal function groups (Table 3, eTable 3 in the ESM).
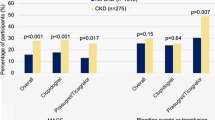
The average duration of DAPT ranged from 16.7 months in the eGFR < 30 mL/min/1.73 m2 category to 19.8 months in the eGFR ≥ 90 category (p < 0.0001). Use of DAPT during long-term follow-up declined consistently across the eGFR groups and declined over time (Fig. 1). At 12 months, 57.0% of patients with eGFR < 30 mL/min/1.73 m2 and 78.0% of those with eGFR ≥ 90 mL/min/1.73 m2 were still receiving DAPT. At the final follow-up visit, 38.8% of patients in the eGFR < 30 category continued to receive DAPT compared with 57.5% in the highest eGFR group. A similar pattern was observed across the three ACS diagnostic groups (eTable 3 in the ESM).
Rates of death, nonfatal MI, nonfatal stroke, heart failure, and bleeding during long-term follow-up were also higher in patients with lower eGFR, although overall coronary revascularization rates did not differ significantly between the categories (Table 3, eTable 3 in the ESM).
In the unadjusted proportional hazards model, there was a clear trend for lower eGFR levels to be associated with an increased risk of the composite cardiovascular endpoint and individual endpoints of death, MI, ischemic stroke, bleeding, and major bleeding over the 2-year follow-up period (Fig. 2). The crude HR for all-cause death, in particular, was much higher when comparing patients with eGFR < 30 versus those with eGFR ≥ 90 mL/min/1.73 m2 (10.71 [95% CI 8.53–13.45]) than the equivalent comparison for patients with eGFR 60–89 mL/min/1.73 m2 (2.20 [95% CI 1.88–2.58]). After adjustment for potential confounding factors, the HRs showed a similar consistent trend across the eGFR groups for all outcomes except major bleeding.

taken from a Cox proportional hazards model for time to the endpoint in question, containing eGFR (< 30, 30–59, 60–89, and ≥ 90) as a covariate. CABG coronary artery bypass graft, CI confidence interval, eGFR estimated glomerular filtration rate, EQ-5D EuroQol 5-Dimensions, HR hazard ratio, MI myocardial infarction, NSTEMI non-ST-segment elevation myocardial infarction, PCI percutaneous coronary intervention, STEMI ST-segment elevation myocardial infarction, UA unstable angina
Risk of cardiovascular events by eGFR category, relative to eGFR ≥ 90 mL/min/1.73 m2, during the 2-year follow-up period: a eGFR < 30, b eGFR 30–59, and c eGFR 60–89 mL/min/1.73 m2. *Model adjusted for age (per 10 years), sex, final diagnosis of index event (UA/STEMI/NSTEMI), EQ-5D overall health state at discharge, in-hospital cardiovascular events, previous chronic obstructive pulmonary disease/lung disease, previous peripheral vascular disease, prior PCI or CABG, admission hemoglobin (< 13/≥ 13 g/dL), and diuretics at discharge. HRs and 95% CIs relative to eGFR ≥ 90 mL/min/1.73 m2,
4 Discussion
These data from EPICOR and EPICOR Asia indicate that patients with renal dysfunction less frequently underwent early PCI or CABG and were less likely to be discharged on DAPT, with decreasing use across the eGFR groups as renal function declined. The results are in keeping with those of other studies [4, 15,16,17,18] and despite the known association between CKD and increased risk of CVD, ACS, and subsequent in-hospital events or death [23,24,25,26]. We also showed that in-hospital and long-term mortality, cardiovascular and bleeding event rates were higher in patients with CKD, particularly in those with eGFR < 30 mL/min/1.73 m2. Thus, although patients with poorer renal function were less likely to receive DAPT at discharge and more likely to discontinue it early, they still had higher bleeding rates than those with “normal” renal function. This is the most likely reason for the apparent “undertreatment” with antithrombotics in this population.
Less than 20% of the ACS survivors enrolled in these studies had eGFR < 60, which is somewhat lower than the 31–43% reported previously in the ACTION registry, although it should be noted that the ACTION cohort included patients who died in hospital [4]. Furthermore, it was only in the lowest eGFR group (< 30 mL/min/1.73 m2) that the proportion of patients with STEMI was exceeded by those with NSTEMI. These discrepancies may result from the large and diverse population included in our cohort [27, 28]. Overall, however, our population characteristics aligned with those observed elsewhere in terms of enhanced cardiovascular risk [1,2,3,4,5,6].
There is limited guidance on the use of PCI and antiplatelet therapy in patients with ACS and CKD. Neither the 2013 American College of Cardiology Foundation/American Heart Association (AHA) STEMI guidelines nor the 2015 American College of Cardiology (ACC)/AHA/Society for Cardiovascular Angiography and Interventions guidelines for use of PCI in patients with STEMI refer to renal function [29, 30]. European Society of Cardiology guidelines for the management of patients with STEMI and NSTE-ACS (specifically referred to in the guidelines’ web-addenda) state that choice and dose of antithrombotic agents need to be considered carefully in the context of bleeding risk in patients with renal dysfunction, but no dose adjustment is required for aspirin or the P2Y12 inhibitors clopidogrel, prasugrel, and ticagrelor [7, 8]. The authors of a recent meta-analysis and review of prasugrel or ticagrelor in patients with CKD and ACS concluded that the newer antiplatelet agents were associated with a reduced rate of major adverse cardiovascular events compared with clopidogrel, without an increased bleeding risk [31, 32]. AHA/ACC NSTE-ACS guidelines note that hypo-responsiveness to clopidogrel may be an issue in patients with CKD and that ticagrelor may be a better alternative based on trial results [33]. Data from the PLATO (Platelet Inhibition and Patient Outcomes) trial confirmed the benefit of ticagrelor over clopidogrel to reduce the risk of further cardiovascular events in the 12 months following an ACS in patients with CKD [34, 35]. Nevertheless, evidence from the TRANSLATE-ACS (Treatment with ADP Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome) study suggested that the risk of moderate or severe bleeding with higher-potency P2Y12 inhibitors increased with the severity of CKD and that treatment interruptions or a switch to clopidogrel may be more likely in those with more advanced disease [36].
Our analysis showed that DAPT duration during follow-up was shorter in patients with CKD and decreased across the four categories of eGFR at each of the 6-month time points over the 2-year period, but still exceeded 12 months in a significant proportion of patients. A similar pattern was observed in the overall EPICOR and EPICOR Asia studies, with the majority of patients continuing to receive DAPT beyond 12 months [37,38,39,40]. Recent data from the large SWEDEHEART (Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies) registry showed that prolonged use of DAPT (compared with 3 months, duration) following an ACS is associated with a lower risk of death, stroke, and MI in patients with or without CKD, with a similar bleeding risk in each case [14].
During the 2-year follow-up period in EPICOR and EPICOR Asia, patients with lower eGFR had higher rates of adverse outcomes, including death, nonfatal MI, nonfatal stroke, and bleeding. For example, 2-year mortality was 22.1% in patients in the lowest renal function group compared with 2.4% in the highest group and 5.2% in the overall EPICOR Asia population [41]. HRs for the composite and individual cardiovascular endpoints and death were higher in patients with poorer renal function on both unadjusted and adjusted analysis. Similar findings have been reported elsewhere in Asian populations [42, 43]. Of note, this evidence is relevant because Asian populations have historically been under-represented in both observational and randomized studies [44].
There are several limitations to this analysis of data from the EPICOR and EPICOR Asia studies [19, 27, 37,38,39,40,41, 45,46,47,48,49,50,51,52]. First, considerations inherent to the analysis of observational data must be taken into account, such as a potential patient selection bias in terms of differences in DAPT patterns and clinical outcomes across the eGFR groups. Second, the studies excluded data from non-survivors of the index event and from patients transferred to a non-participating hospital [45]. Another limitation is the lack of information on specific reasons for non-use of antithrombotic agents at discharge or for discontinuation of DAPT during long-term follow-up [38]. Third, the study did not track how renal function changes over time nor, therefore, the degree to which this impacted on further treatment decisions (e.g., stopping DAPT or undergoing subsequent intervention) or long-term outcomes. Finally, the use of quality indicators [52] in this patient subgroup has not been validated and may be challenging.
In conclusion, the presence of CKD in patients with ACS in the EPICOR and EPICOR Asia studies was associated with less aggressive cardiovascular management and an increased risk of cardiovascular events and bleeding, both in hospital and during long-term follow-up, with a consistent pattern across the three ACS diagnostic categories. Patients with ACS and renal dysfunction, therefore, require careful assessment and tailored short- and long-term antithrombotic management, including selection of appropriate oral antiplatelet therapy, to minimize the risk of adverse clinical events. This recommendation may be particularly pertinent for patients in geographical areas with an apparently higher prevalence of renal dysfunction (Thailand/Vietnam/Malaysia and Latin America) and in patients with poorer socio-economic indicators, such as rural residence, no formal education, and lack of medical insurance. Future real-world evidence studies looking at long-term antithrombotic management strategies and outcomes of patients with ACS should be designed to take such issues into consideration.
References
Kang YU, Jeong MH, Kim SW. Impact of renal dysfunction on clinical outcomes of acute coronary syndrome. Yonsei Med J. 2009;50(4):537–45.
Washam JB, Herzog CA, Beitelshees AL, Cohen MG, Henry TD, Kapur NK, et al. Pharmacotherapy in chronic kidney disease patients presenting with acute coronary syndrome: a scientific statement from the American Heart Association. Circulation. 2015;131(12):1123–49.
Huang CC, Chen JW. Contemporary management of coronary artery disease and acute coronary syndrome in patients with chronic kidney disease and end-stage renal disease. Acta Cardiol Sin. 2013;29(2):132–41.
Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121(3):357–65.
Goldenberg I, Subirana I, Boyko V, Vila J, Elosua R, Permanyer-Miralda G, et al. Relation between renal function and outcomes in patients with non-ST-segment elevation acute coronary syndrome: real-world data from the European Public Health Outcome Research and Indicators Collection Project. Arch Intern Med. 2010;170(10):888–95.
Dohi T, Kasai T, Miyauchi K, Takasu K, Kajimoto K, Kubota N, et al. Prognostic impact of chronic kidney disease on 10-year clinical outcomes among patients with acute coronary syndrome. J Cardiol. 2012;60(6):438–42.
Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020. https://doi.org/10.1093/eurheartj/ehaa575
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Basra SS, Tsai P, Lakkis NM. Safety and efficacy of antiplatelet and antithrombotic therapy in acute coronary syndrome patients with chronic kidney disease. J Am Coll Cardiol. 2011;58(22):2263–9.
Rossello X, Ariti C, Pocock SJ, Ferreira JP, Girerd N, McMurray JJV, et al. Impact of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with heart failure and left-ventricular systolic dysfunction: an individual patient-level meta-analysis of three randomized-controlled trials. Clin Res Cardiol. 2019;108(5):477–86.
Huang HD, Alam M, Hamzeh I, Virani S, Deswal A, Aguilar D, et al. Patients with severe chronic kidney disease benefit from early revascularization after acute coronary syndrome. Int J Cardiol. 2013;168(4):3741–6.
Shaw C, Nitsch D, Lee J, Fogarty D, Sharpe CC. Impact of an early invasive strategy versus conservative strategy for unstable angina and non-ST elevation acute coronary syndrome in patients with chronic kidney disease: a systematic review. PLoS ONE. 2016;11(5):e0153478.
Szummer K, Lundman P, Jacobson SH, Schon S, Lindback J, Stenestrand U, et al. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009;120(10):851–8.
Carrero JJ, Varenhorst C, Jensevik K, Szummer K, Lagerqvist B, Evans M, et al. Long-term versus short-term dual antiplatelet therapy was similarly associated with a lower risk of death, stroke, or infarction in patients with acute coronary syndrome regardless of underlying kidney disease. Kidney Int. 2017;91(1):216–26.
Chu CY, Lin TH, Lai WT. The management and prognostic factors of acute coronary syndrome: evidence from the Taiwan Acute Coronary Syndrome Full Spectrum Registry. Acta Cardiol Sin. 2017;33(4):329–38.
Rhee JW, Wiviott SD, Scirica BM, Gibson CM, Murphy SA, Bonaca MP, et al. Clinical features, use of evidence-based therapies, and cardiovascular outcomes among patients with chronic kidney disease following non-ST-elevation acute coronary syndrome. Clin Cardiol. 2014;37(6):350–6.
Khedri M, Szummer K, Carrero JJ, Jernberg T, Evans M, Jacobson SH, et al. Systematic underutilisation of secondary preventive drugs in patients with acute coronary syndrome and reduced renal function. Eur J Prev Cardiol. 2017;24(7):724–34.
Rozenbaum Z, Benchetrit S, Minha S, Neuman Y, Shlezinger M, Goldenberg I, et al. The effect of admission renal function on the treatment and outcome of patients with acute coronary syndrome. Cardiorenal Med. 2017;7(3):169–78.
Rossello X, Bueno H, Pocock SJ, Van de Werf F, Danchin N, Annemans L, et al. Predictors of all-cause mortality and ischemic events within and beyond 1 year after an acute coronary syndrome: results from the EPICOR registry. Clin Cardiol. 2019;42(1):111–9.
Bueno H, Danchin N, Tafalla M, Bernaud C, Annemans L, Van de Werf F. EPICOR (long-tErm follow-up of antithrombotic management Patterns In acute CORonary syndrome patients) study: rationale, design, and baseline characteristics. Am Heart J. 2013;165(1):8–14.
Huo Y, Lee SWL, Sawhney JPS, Kim H-S, Krittayaphong R, Nhan VT, et al. Rationale, design, and baseline characteristics of the EPICOR Asia study (Long-tErm follow-uP of antithrombotic management patterns In Acute CORonary Syndrome patients in Asia). Clin Cardiol. 2015;38(9):511–9.
Soares AA, Eyff TF, Campani RB, Ritter L, Camargo JL, Silveiro SP. Glomerular filtration rate measurement and prediction equations. Clin Chem Lab Med. 2009;47(9):1023–32.
Gibson CM, Pinto DS, Murphy SA, Morrow DA, Hobbach HP, Wiviott SD, et al. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol. 2003;42(9):1535–43.
Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–95.
Santopinto JJ, Fox KA, Goldberg RJ, Budaj A, Pinero G, Avezum A, et al. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE). Heart. 2003;89(9):1003–8.
Lee SH, Kim YJ, Kim W, Park JS, Shin DG, Hur SH, et al. Clinical outcomes and therapeutic strategy in patients with acute myocardial infarction according to renal function: data from the Korean Acute Myocardial Infarction Registry. Circ J. 2008;72(9):1410–8.
Rossello X, Huo Y, Pocock S, Van de Werf F, Chin CT, Danchin N, et al. Global geographical variations in ST-segment elevation myocardial infarction management and post-discharge mortality. Int J Cardiol. 2017;245:27–34.
Bueno H, Rossello X, Pocock S, Van de Werf F, Chin CT, Danchin N, et al. Regional variations in hospital management and post-discharge mortality in patients with non-ST-segment elevation acute coronary syndrome. Clin Res Cardiol. 2018;107(9):836–44.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI Focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention and the 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133(11):1135–47.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;61(4):485–510.
Bonello L, Laine M, Lemesle G, Puymirat E, Dabry T, Thuny F, et al. Meta-analysis of potent P2Y12-ADP receptor antagonist therapy compared to clopidogrel therapy in acute coronary syndrome patients with chronic kidney disease. Thromb Haemost. 2018;118(10):1839–46.
Bonello L, Angiolillo DJ, Aradi D, Sibbing D. P2Y(12)-ADP receptor blockade in chronic kidney disease patients with acute coronary syndromes. Circulation. 2018;138(15):1582–96.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130(25):e344–426.
James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122(11):1056–67.
Franchi F, James SK, Ghukasyan Lakic T, Budaj AJ, Cornel JH, Katus HA, et al. Impact of diabetes mellitus and chronic kidney disease on cardiovascular outcomes and platelet P2Y(12) receptor antagonist effects in patients with acute coronary syndromes: insights from the PLATO trial. J Am Heart Assoc. 2019;8(6):e011139.
Rymer JA, Kaltenbach LA, Doll JA, Messenger JC, Peterson ED, Wang TY. Safety of dual-antiplatelet therapy after myocardial infarction among patients with chronic kidney disease. J Am Heart Assoc. 2019;8(10):e012236.
Zheng B, Huo Y, Lee SW, Sawhney JPS, Kim HS, Krittayaphong R, et al. Long-term antithrombotic management patterns in Asian patients with acute coronary syndrome: 2-year observations from the EPICOR Asia study. Clin Cardiol. 2020;43(9):999–1008.
Bueno H, Pocock S, Danchin N, Annemans L, Gregson J, Medina J, et al. International patterns of dual antiplatelet therapy duration after acute coronary syndromes. Heart. 2017;103(2):132–8.
Zhang S, Wang W, Sawhney JPS, Krittayaphong R, Kim HS, Nhan VT, et al. Antithrombotic management and long-term outcomes following percutaneous coronary intervention for acute coronary syndrome in Asia. Int J Cardiol. 2020;310:16–22.
Zou Y, Yang S, Wang S, Lv B, Xiu L, Li L, et al. Prolonged dual antiplatelet therapy in patients with non-ST-segment elevation myocardial infarction: 2-year findings from EPICOR Asia. Clin Cardiol. 2020;43(4):346–54.
Huo Y, Lee SW, Sawhney JPS, Kim HS, Krittayaphong R, Pocock SJ, et al. Two-year outcomes post-discharge in Asian patients with acute coronary syndrome: findings from the EPICOR Asia study. Int J Cardiol. 2020;315:1–8.
Li C, Hu D, Shi X, Li L, Yang J, Song L, et al. A multicentre prospective evaluation of the impact of renal insufficiency on in-hospital and long-term mortality of patients with acute ST-elevation myocardial infarction. Chin Med J (Engl). 2015;128(1):1–6.
Otsuka K, Shimada K, Katayama H, Nakamura H, Ishikawa H, Takeda H, et al. Prognostic significance of renal dysfunction and its change pattern on outcomes in patients with acute coronary syndrome treated with emergent percutaneous coronary intervention. Heart Vessels. 2019;34(5):735–44.
Lüscher TF, Miller VM, Bairey Merz CN, Crea F. Diversity is richness: why data reporting according to sex, age, and ethnicity matters. Eur Heart J. 2020;41(33):3117–21.
Sinnaeve PR, Zeymer U, Bueno H, Danchin N, Medina J, Sanchez-Covisa J, et al. Contemporary inter-hospital transfer patterns for the management of acute coronary syndrome patients: findings from the EPICOR study. Eur Heart J Acute Cardiovasc Care. 2015;4(3):254–62.
Annemans L, Danchin N, Van de Werf F, Pocock S, Licour M, Medina J, et al. Prehospital and in-hospital use of healthcare resources in patients surviving acute coronary syndromes: an analysis of the EPICOR registry. Open Heart. 2016;3(1):e000347.
Bueno H, Martin AR. Long-term cardiovascular risk after acute coronary syndrome, an ongoing challenge. Rev Esp Cardiol (Engl Ed). 2016;69(1):1–2.
Ong TK, Lee SW-L, Sawhney JPS, Kim H-S, Krittayaphong R, Nhan VT, et al. Management of acute coronary syndrome and the importance of hospital access: findings from the EPICOR Asia study. Eur. J Cardiovasc Med. 2016;4(1):522–30.
Chin CT, Ong TK, Krittayaphong R, Lee SW, Sawhney JPS, Kim HS, et al. Characteristics and outcomes of medically managed patients with non-ST-segment elevation acute coronary syndromes: insights from the multinational EPICOR Asia study. Int J Cardiol. 2017;243:15–20.
Jan S, Lee SW, Sawhney JP, Ong TK, Chin CT, Kim HS, et al. Catastrophic health expenditure on acute coronary events in Asia: a prospective study. Bull WHO. 2016;94(3):193–200.
Bueno H, Rossello X, Pocock SJ, Van de Werf F, Chin CT, Danchin N, et al. In-hospital coronary revascularization rates and post-discharge mortality risk in non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2019;74(11):1454–61.
Rossello X, Medina J, Pocock S, Van de Werf F, Chin CT, Danchin N, et al. Assessment of quality indicators for acute myocardial infarction management in 28 countries and use of composite quality indicators for benchmarking. Eur Heart J Acute Cardiovasc Care. 2020. https://doi.org/10.1177/2048872620911853
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
EPICOR and EPICOR Asia were funded by AstraZeneca. As they were noninterventional studies, no drugs were supplied or funded.
Conflicts of Interest
F. Van de Werf has received consulting fees and research grants from Boehringer Ingelheim and Merck and consulting fees from Roche, Sanofi-Aventis, AstraZeneca, and The Medicines Company. S.J. Pocock has received research funding from AstraZeneca. C.T. Chin has received research support from Eli Lilly and honoraria from Medtronic and has been a consultant or advisory board member for AstraZeneca. A.M. Vega is a former employee of AstraZeneca. J. Medina is an employee of AstraZeneca. H. Bueno receives research funding from the Instituto de Salud Carlos III, Spain (PIE16/00021 and PI17/01799), Sociedad Española de Cardiología, AstraZeneca, Bayer, BMS, and Novartis; has received consulting fees from AstraZeneca, Bayer, BMS-Pfizer, and Novartis; and speaking fees or support for attending scientific meetings from Amgen, AstraZeneca, Bayer, BMS-Pfizer, Novartis, and MEDSCAPE-the heart.org. X. Rossello has received support from the SEC-CNIC CARDIOJOVEN fellowship program. Y. Huo, Y. Han, S. W.-L. Lee, Y. Li, and J. Jiang have no conflicts of interest that are directly relevant to the content of this article. AstraZeneca reviewed the manuscript during development and could make suggestions; however, final content, opinions, conclusions, and interpretation of the data are the responsibility of the authors. Professor Huo had full access to all of the data in the studies and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Availability of data and material
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics approval
In both EPICOR and EPICOR Asia, the final study protocol was approved by the ethics committees of participating centers according to local regulations. The studies were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent
All patients provided written informed consent at discharge from hospital and completed a contact order form agreeing to be contacted by telephone for regular follow-up interviews post discharge.
Author contributions
Conceptualization: Yong Huo, Héctor Bueno, Stuart Pocock, and Ana Maria Vega (equal). Formal analysis: Stuart Pocock and Richard Cairns of Worldwide Clinical Trials (equal). Funding acquisition: Ana Maria Vega and Jesús Medina (equal). Investigation: Yong Huo, Frans Van de Werf, Yaling Han, Xavier Rossello, Chee Tang Chin, Stephen W-L Lee, Yi Li, Jie Jiang, and Héctor Bueno (equal). Methodology: Yong Huo, Héctor Bueno, Stuart Pocock, Ana Maria Vega, and Jesús Medina (equal). Project administration: Yong Huo and Héctor Bueno (equal). Resources: Ana Maria Vega and Jesús Medina (equal). Supervision: Yong Huo and Héctor Bueno (equal). Validation: Yong Huo, Héctor Bueno, and Stuart Pocock (equal). Visualization: Yong Huo, Héctor Bueno, and Stuart Pocock (equal). Writing – original draft: Yong Huo, Héctor Bueno, Stuart Pocock, and Xavier Rossello (equal). Writing – review and editing: All authors (equal).
Acknowledgements
The authors thank Richard Cairns of Worldwide Clinical Trials (Nottingham, UK) for statistical analysis. Editorial support was provided by Carl V Felton PhD of Prime Global (Knutsford, Cheshire, UK), according to Good Publication Practice guidelines, and was funded by AstraZeneca.
Supplementary information
Below is the link to the supplementary information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huo, Y., Van de Werf, F., Han, Y. et al. Long-Term Antithrombotic Therapy and Clinical Outcomes in Patients with Acute Coronary Syndrome and Renal Impairment: Insights from EPICOR and EPICOR Asia. Am J Cardiovasc Drugs 21, 471–482 (2021). https://doi.org/10.1007/s40256-020-00447-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-020-00447-5