Abstract
Objective
Apixaban is a substrate of cytochrome P450 3A4 (CYP3A4) and P-glycoprotein. The effects of rifampin, a strong inducer of CYP3A4 and P-glycoprotein, on the pharmacokinetics of oral and intravenous apixaban were evaluated in an open-label, randomized, sequential crossover study.
Methods
Twenty healthy participants received single doses of apixaban 5 mg intravenously on day 1 and 10 mg orally on day 3, followed by rifampin 600 mg once daily on days 5–15. Finally, participants received single doses of apixaban 5 mg intravenously and 10 mg orally separately on days 12 and 14 in one of two randomized sequences.
Results
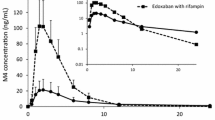
Apixaban, given intravenously and orally, was safe and well tolerated when administered in the presence and absence of rifampin. Apixaban absolute oral bioavailability was 49 % when administered alone and 39 % following induction by rifampin. Rifampin reduced apixaban area under the plasma concentration–time curve from time zero to infinity (AUC∞) by 39 % after intravenous administration and by 54 % after oral administration. Rifampin induction increased mean clearance by 1.6-fold for intravenous apixaban and mean apparent clearance by 2.1-fold for oral apixaban, indicating rifampin affected both pre-systemic and systemic apixaban elimination pathways.
Conclusion
Co-administration of apixaban with rifampin reduced apixaban exposure via both decreased bioavailability and increased systemic clearance.


Similar content being viewed by others
References
Pinto DJ, Orwat MJ, Koch S, et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem. 2007;50:5339–56.
Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008;6:820–9.
Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–98.
Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–15.
Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Agnelli G, Buller HR, Cohen, et al. AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808.
Agnelli G, Buller HR, Cohen A, et al. AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708.
Frost C, Nepal S, Wang J, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76:776–86.
Frost C, Wang J, Nepal S, et al. Apixaban, an oral, direct factor Xa inhibitor: single-dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75:476–87.
Frost C, Yu Z, Nepal S, et al. Apixaban, a direct factor Xa inhibitor: single-dose pharmacokinetics and pharmacodynamics of an intravenous formulation [abstract 148]. J Clin Pharmacol. 2008;48:1132.
Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
Wang L, He K, Maxwell B, et al. Tissue distribution and elimination of [14C]apixaban in rats. Drug Metab Dispos. 2011;39:256–64.
Zhang D, Frost CE, He K, et al. Investigating the enteroenteric recirculation of apixaban, a factor Xa inhibitor: administration of activated charcoal to bile duct-cannulated rats and dogs receiving an intravenous dose and use of drug transporter knockout rats. Drug Metab Dispos. 2013;41:906–15.
Zhang D, He K, Raghavan N, et al. Comparative metabolism of 14C-labeled apixaban in mice, rats, rabbits, dogs, and humans. Drug Metab Dispos. 2009;37:1738–48.
Wang L, Raghavan N, He K, et al. Sulfation of o-demethyl apixaban: enzyme identification and species comparison. Drug Metab Dispos. 2009;37:802–8.
Wang L, Zhang D, Raghavan N, et al. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos. 2010;38:448–58.
He K, Luettgen JM, Zhang D, et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur J Drug Metab Pharmacokinet. 2011;36:129–39.
Zhang D, He K, Herbst JJ, et al. Characterization of efflux transporters involved in distribution and disposition of apixaban. Drug Metab Dispos. 2013;41:827–35.
Giacomini KM, Huang SM, Tweedie DJ, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36.
Benet LZ, Cummins CL. The drug efflux-metabolism alliance: biochemical aspects. Adv Drug Deliv Rev. 2001;50(Suppl 1):S3–11.
Frost CE, Byon W, Song Y, et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol. 2015;79:838–46.
Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147–53.
Sanofi-Aventis U.S. LLC. Rifadin® (rifampin) package insert. 2013. http://products.sanofi.us/rifadin/Rifadin.pdf. Accessed 2 Feb 2015.
Kolars JC, Schmiedlin-Ren P, Schuetz JD, et al. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90:1871–8.
Pursley J, Shen JX, Schuster A, et al. LC-MS/MS determination of apixaban (BMS-562247) and its major metabolite in human plasma: an application of polarity switching and monolithic HPLC column. Bioanalysis. 2014;6:2071–82.
Perrier D, Gibaldi M. General derivation of the equation for time to reach a certain fraction of steady state. J Pharm Sci. 1982;71:474–5.
Niemi M, Backman JT, Fromm MF, et al. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50.
Kageyama M, Fukushima K, Togawa T, et al. Relationship between excretion clearance of rhodamine 123 and P-glycoprotein (Pgp) expression induced by representative Pgp inducers. Biol Pharm Bull. 2006;29:779–84.
Yamada S, Uasui-Furukori N, Akamine Y, et al. Effects of the P-glycoprotein inducer carbamazepine on fexofenadine pharmacokinetics. Ther Drug Monit. 2009;31:764–8.
Wang X, Mondal S, Wang J, et al. Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am J Cardiovasc Drugs. 2014;14:147–54.
Yamahira N, Frost C, Fukase H, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of apixaban in healthy Japanese male subjects. Int J Clin Pharmacol Ther. 2014;52:564–73.
Leil TA, Frost C, Wang X, Pfister M, LaCreta F. Model-based exposure-response analysis of apixaban to quantify bleeding risk in special populations of subjects undergoing orthopedic surgery. CPT Pharmacomet Syst Pharmacol. 2014;3:e136.
Frost CE, Song Y, Shenker A, et al. Effects of age and sex on the single-dose pharmacokinetics and pharmacodynamics of apixaban. Clin Pharmacokinet. 2015;54:651–62.
Leucuta SE, Vlase L. Pharmacokinetics and metabolic drug interactions. Curr Clin Pharmacol. 2006;1:5–20.
Food and Drug Administration. Guidance for industry. Drug interaction studies—study design, data analysis and implications for dosing and labeling: draft guidance. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf. Accessed 2 Feb 2015.
Backman JT, Kivistö KT, Olkkola KT, et al. The area under the plasma concentration–time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol. 1998;54:53–8.
Link B, Haschke M, Grignaschi N, et al. Pharmacokinetics of intravenous and oral midazolam in plasma and saliva in humans: usefulness of saliva as matrix for CYP3A phenotyping. Br J Clin Pharmacol. 2008;66:473–84.
Bristol-Myers Squibb, Pfizer EEIG. Eliquis® (apixaban tablets) Summary of product characteristics. 2013 May. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf. Accessed 21 Aug 2015.
Bristol-Myers Squibb. Eliquis (apixaban tablets). Prescribing information. 2014 August. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed 21 Aug 2015.
Acknowledgments
The authors would like to thank Suzette Girgis, Sunil Nepal, Alan Schuster and Tong Li for their contributions to this study. Editorial assistance was provided by Dana Fox, PhD, CMPP (Caudex, New York, NY, USA), and was funded by Bristol-Myers Squibb Company and Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
This study was sponsored by Bristol-Myers Squibb and Pfizer Inc. Blisse Vakkalagadda, Charles Frost, Jessie Wang, Donglu Zhang, Zhigang Yu, Clapton Dias, Andrew Shenker, and Frank LaCreta were all employees of Bristol-Myers Squibb Company at the time of research and received salaries and benefits commensurate with employment; Wonkyung Byon and Rebecca A. Boyd are employees of Pfizer Inc and received salaries and benefits commensurate with employment. All authors have full control of all primary data, and they agree to allow the journal to review their data if requested.
Ethical standards
This study was performed at a single clinical center in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the New England Institutional Review Board (Wellesley, Massachusetts, USA). All participants provided written, informed consent.
Rights and permissions
About this article
Cite this article
Vakkalagadda, B., Frost, C., Byon, W. et al. Effect of Rifampin on the Pharmacokinetics of Apixaban, an Oral Direct Inhibitor of Factor Xa. Am J Cardiovasc Drugs 16, 119–127 (2016). https://doi.org/10.1007/s40256-015-0157-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-015-0157-9