Abstract
Objective
type 2 diabetes, metabolic disorder, is one of the main risk factors for cardiovascular disease, leading to angiogenesis injury. The present study wanted to discover the effect of sodium butyrate (NaB) and voluntary exercise, alone or together, on miR-126 and related proteins in rats with type 2 diabetes.
Methods
thirty-five male Wistar rats (200–250 g) were randomly divided into five groups: control, diabetes, diabetes-NaB, diabetes-exercise, and diabetes-NaB-exercise. Type 2 diabetes was induced by intraperitoneal injection of streptozotocin (35 mg/kg) and high-fat diet. The rats were then administrated NaB (200 mg/kg. ip) or were subjected to voluntary exercise, or combined NaB and voluntary exercise for 8 weeks. MiR-126 expression in the cardiac tissue was determined by real-time PCR, and the SPRED-1 and RAF proteins expression levels were measured by western blot.
Results
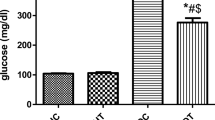
NaB and voluntary exercise up-regulated cardiac miR-126 and RAF expression levels and down-regulated SPRED-1 in cardiac tissue of type 2 diabetic rats. Moreover, the combination of NaB and voluntary exercise amplified their effects on those parameters. Both NaB and voluntary exercise or together markedly modulated serum glucose and HbA1c.
Conclusion
The present findings demonstrated that NaB combined with exercise could improve cardiac angiogenesis by increasing miR-126 and affecting related proteins. Thus, NaB together with voluntary exercise might be a promising intervention for the treatment and prevention of type 2 diabetes.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the first author, upon reasonable request.
Change history
18 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s40200-024-01395-6
References
Teodoro JS, Nunes S, Rolo AP, Reis F, Palmeira CM. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front Physiol. 2019;9:1857. https://doi.org/10.3389/fphys.2018.01857.
Mota RI, Morgan SE, Bahnson EM. Diabetic vasculopathy: macro and microvascular injury. Curr Pathobiol Rep. 2020;8(1):1–14. https://doi.org/10.1007/s40139-020-00205-x.
Strain WD, Paldánius P. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):1–10. https://doi.org/10.1186/s12933-018-0703-2.
Park JJ, Kim S-H, Kim M-A, Chae I-H, Choi D-J, Yoon C-H. Effect of hyperglycemia on myocardial perfusion in diabetic porcine models and humans. J Korean Med Sci. 2019;34(29). https://doi.org/10.3346/jkms.2019.34.e202.
Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49(3):554–60. https://doi.org/10.1016/S0008-6363(00)00228-5.
Regazzi R. MicroRNAs as therapeutic targets for the treatment of diabetes mellitus and its complications. Expert Opin Ther Targets. 2018;22(2):153–60. https://doi.org/10.1080/14728222.2018.1420168.
Chen H, Lan H-Y, Roukos DH, Cho WC. Application of microRNAs in diabetes mellitus. J Endocrinol. 2014;222(1):R1–10. https://doi.org/10.1530/JOE-13-0544.
Barutta F, Bruno G, Matullo G, Chaturvedi N, Grimaldi S, Schalkwijk C, et al. MicroRNA-126 and micro-/macrovascular complications of type 1 diabetes in the EURODIAB Prospective Complications Study. Acta Diabetol. 2017;54(2):133–9. https://doi.org/10.1007/s00592-016-0915-4.
Rawal S, Munasinghe PE, Shindikar A, Paulin J, Cameron V, Manning P, et al. Down-regulation of proangiogenic microRNA-126 and microRNA-132 are early modulators of diabetic cardiac microangiopathy. Cardiovasc Res. 2017;113(1):90–101. https://doi.org/10.1093/cvr/cvw235.
Wang L, Lee AYW, Wigg JP, Peshavariya H, Liu P, Zhang H. miR-126 regulation of angiogenesis in age-related macular degeneration in CNV mouse model. Int J Mol Sci. 2016;17(6):895. https://doi.org/10.3390/ijms17060895.
Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–84. https://doi.org/10.1016/j.devcel.2008.07.008.
Nammian P, Razban V, Tabei S, Asadi-Yousefabad S-L. MicroRNA-126: Dual role in angiogenesis dependent diseases. Curr Pharm Des. 2020;26(38):4883–93. https://doi.org/10.2174/1381612826666200504120737.
Canani RB, Di Costanzo M, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol: WJG. 2011;17(12):1519. https://doi.org/10.3748/wjg.v17.i12.1519.
Du J, Zhang L, Zhuang S, Qin GJ, Zhao TC. HDAC4 degradation mediates HDAC inhibition-induced protective effects against hypoxia/reoxygenation injury. J Cell Physiol. 2015;230(6):1321–31. https://doi.org/10.1002/jcp.24871.
Ye J. Improving insulin sensitivity with HDAC inhibitor. Diabetes. 2013;62(3):685–7. https://doi.org/10.2337/db12-1354.
Bridgeman SC, Northrop W, Melton PE, Ellison GC, Newsholme P, Mamotte CD. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol Res. 2020;160:105174. https://doi.org/10.1016/j.phrs.2020.105174.
Barber TM, Kabisch S, Pfeiffer AF, Weickert MO. The health benefits of dietary fibre. Nutrients. 2020;12(10):3209. https://doi.org/10.3390/nu12103209.
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–17. https://doi.org/10.2337/db08-1637.
Dunford EC, Leclair E, Aiken J, Mandel ER, Haas TL, Birot O, et al. The effects of voluntary exercise and prazosin on capillary rarefaction and metabolism in streptozotocin-induced diabetic male rats. J Appl Physiol. 2017;122(3):492–502. https://doi.org/10.1152/japplphysiol.00762.2016.
Chodari L, Pourheydar B, Dariushnejad H, Jamshidi S, Khalaji N, Ghorbanzadeh V. Testosterone Combined with Voluntary Exercise Attenuates Diabetes-induced Pancreatic Apoptosis in Castrated Diabetic Rats Induced by HFD/STZ. Braz Arch Biol Technol. 2021;64. https://doi.org/10.1590/1678-4324-2021200037.
Buniam J, Chukijrungroat N, Khamphaya T, Weerachayaphorn J, Saengsirisuwan V. Estrogen and voluntary exercise attenuate cardiometabolic syndrome and hepatic steatosis in ovariectomized rats fed a high-fat high-fructose diet. Am J Physiol-Endocrinol Metab. 2019;316(5):E908–21. https://doi.org/10.1152/ajpendo.00466.2018.
Manzanares G, Brito-da-Silva G, Gandra P. Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res. 2018;52(1). https://doi.org/10.1590/1414-431X20187830.
Zhang M, Lv X-Y, Li J, Xu Z-G, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008. https://doi.org/10.1155/2008/704045.
Adeyanju OA, Badejogbin OC, Areola DE, Olaniyi KS, Dibia C, Soetan OA, et al. Sodium butyrate arrests pancreato-hepatic synchronous uric acid and lipid dysmetabolism in high fat diet fed Wistar rats. Biomed Pharmacother. 2021;133:110994. https://doi.org/10.1016/j.biopha.2020.110994.
Viigimaa M, Sachinidis A, Toumpourleka M, Koutsampasopoulos K, Alliksoo S, Titma T. Macrovascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):110–6. https://doi.org/10.2174/1570161117666190405165151.
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. https://doi.org/10.1016/j.metabol.2021.154766.
Kir D, Schnettler E, Modi S, Ramakrishnan S. Regulation of angiogenesis by microRNAs in cardiovascular diseases. Angiogenesis. 2018;21(4):699–710. https://doi.org/10.1007/s10456-018-9632-7.
Wang M, Zhang W, Zhang L, Wang L, Li J, Shu C, et al. Roles of MicroRNAs in Peripheral Artery In-Stent Restenosis after Endovascular Treatment. Biomed Res Int. 2021. https://doi.org/10.1155/2021/9935671.
Sakai H, Sato K, Ito K, Kosugi I, Kiyama M, Kon R, et al. Inhibition of Spred/Sprouty Expression in the Skin of a Contact Dermatitis-Like Model. Biol Pharm Bull. 2022;45(8):1208–12. https://doi.org/10.1248/bpb.b22-00279.
Gong J, Yan Z, Liu Q. Progress in experimental research on SPRED protein family. J Int Med Res. 2020;48(8):1–14. https://doi.org/10.1177/0300060520929170.
Lew JKS, Pearson JT, Schwenke DO, Katare R. Exercise mediated protection of diabetic heart through modulation of microRNA mediated molecular pathways. Cardiovasc Diabetol. 2017;16(1):1–20. https://doi.org/10.1186/s12933-016-0484-4.
Sabzevari Rad R, Shirvani H, Mahmoodzadeh Hosseini H, Shamsoddini A, Samadi M. Micro RNA-126 promoting angiogenesis in diabetic heart by VEGF/Spred-1/Raf-1 pathway: effects of high-intensity interval training. J Diabetes Metab Disord. 2020;19(2):1089–96. https://doi.org/10.1007/s40200-020-00610-4.
Akbari J, Shirvani H, Shamsoddini A, Bazgir B, Samadi M. Investigation of expression of myocardial miR-126, miR-29a and miR-222 as a potential marker in STZ-induced diabetic rats following interval and continuous exercise training. J Diabetes Metab Disord. 2022:1–7. https://doi.org/10.1007/s40200-021-00957-2.
Song W, Liang Q, Cai M, Tian Z. HIF-1α-induced up-regulation of microRNA-126 contributes to the effectiveness of exercise training on myocardial angiogenesis in myocardial infarction rats. J Cell Mol Med. 2020;24(22):12970–9. https://doi.org/10.1111/jcmm.15892.
Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. BioMed Res Int. 2015. https://doi.org/10.1155/2015/549412.
Wu J, Jiang Z, Zhang H, Liang W, Huang W, Zhang H, et al. Sodium butyrate attenuates diabetes-induced aortic endothelial dysfunction via P300-mediated transcriptional activation of Nrf2. Free Radical Biol Med. 2018;124:454–65. https://doi.org/10.1016/j.freeradbiomed.2018.06.034.
Liu L, Chen Y, Wu Q, Shu A, Sun J. Sodium Butyrate Attenuated Diabetes-Induced Intestinal Inflammation by Modulating Gut Microbiota. Evid-Based Complement Altern Med. 2022. https://doi.org/10.1155/2022/4646245.
Zhang W-Q, Zhao T-T, Gui D-K, Gao C-L, Gu J-L, Gan W-J, et al. Sodium butyrate improves liver glycogen metabolism in type 2 diabetes mellitus. J Agric Food Chem. 2019;67(27):7694–705. https://doi.org/10.1021/acs.jafc.9b02083.
Castro PR, Bittencourt LFF, Larochelle S, Andrade SP, Mackay CR, Slevin M, et al. GPR43 regulates sodium butyrate-induced angiogenesis and matrix remodeling. Am J Physiol-Heart Circ Physiol. 2021;320(3):H1066–79. https://doi.org/10.1152/ajpheart.00515.2019.
Funding
This work was supported by the National Institute for Medical Research Development (NIMAD), Tehran, Iran [972752].
Author information
Authors and Affiliations
Contributions
Hassan Dariushnejad: Conceptualization, Writing original draft, Data collection. Neda Roshanravan: Writing original draft, Data collection. Lale Pirzeh: Visualization, Validation, Data collection. Mostafa Cheraghi: Data collection. Vajihe Ghorbanzadeh: Writing—original draft, Project administration, Supervision, Visualization, Validation, Writing—review & editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Disclosure
The authors declare that there is no conflict of interest.
Ethical issues
The procedure for this study was prearranged to follow the guidelines of NIH, and be in agreement with Ethics Committee for the Use of Animals in National Institute for Medical Research Development in Iran (IR.NIMAD.REC.1397.509).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original publication of the article, the affiliation of the third author Lale Pirzeh was published incorrectly as “Institute for Vascular Signaling, Center for Molecular Medicine, Johann Wolfgang Goethe University Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfort am Main, Germany”. The corrected affiliation should read as 48A, Auf dem Mühlberg, 60599 Frankfurt am Main.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dariushnejad, H., Roshanravan, N., Pirzeh, L. et al. Cardiac angiogenesis enhances by activating Mir-126 and related target proteins in type 2 diabetic rats: Rescue combination effect of Sodium butyrate and voluntary exercise therapy. J Diabetes Metab Disord 22, 753–761 (2023). https://doi.org/10.1007/s40200-023-01198-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01198-1