Abstract
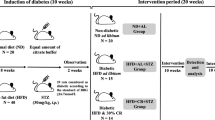
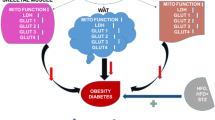
Reduced activity of glucose transporter type 4 isoform (GLUT-4), an insulin-sensitive glucose transporter distributed on the adipocytes, is associated with impaired insulin signaling. Insulin resistance resulting from alteration in glucose transport is responsible for exacerbating the emergence of metabolic abnormalities. The present study aimed to investigate the effects of the antidote gallic acid (GA) on expression-related changes in GLUT-4 and insulin receptor substrate-1 (IRS-1) in the visceral adipose tissue and on the subsequent development of insulin resistance in a high-fat diet (HFD)–induced obesity animal model. Methods: Twenty-four female Swiss albino mice were used and separated into the following four groups (six animals in each group): control group (standard pellet diet), HFD group, (60% HFD), HFD + GA group (60% HFD and GA 50 mg/kg body weight for 60 days), and GA group (GA 50 mg/kg body weight for 60 days). The effect of HFD on serum glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL), low-density lipoprotein (LDL) cholesterol, and insulin was evaluated. Additionally, homeostasis model assessment for insulin resistance (HOMA-IR) and glucose tolerance test (GTT) was performed. The serum antioxidative profile, which comprises oxidative parameters (superoxide dismutase [SOD], catalase [CAT], and glutathione peroxidase [GPx]) was measured. The effectiveness of GA against HFD-induced alteration in GLUT-4 and IRS-1 expression was also evaluated. Results: The experimental group that fed on GA + HFD had improved levels of serum triglycerides (p˂0.001), cholesterol (p˂0.05), and LDL cholesterol. GA administration also significantly improved hyperinsulinemia and HOMA-IR index (p˂0.001) in HFD mice. GA improved GTT results (p˂0.05); activity of SOD, CAT, and GPx (p˂0.05); and upregulated mRNA expression of GLUT-4 and IRS-1(p˂0.05) in the visceral adipose tissue in the HFD + GA experimental group. Conclusion: A link exists between insulin resistance, GLUT-4, and IRS-1 expression in the adipose tissue, and the initiation of metabolic syndrome, a condition characterized by obesity. GA may promote insulin signaling, glucose uptake, and lipid metabolism in the adipose tissues by mitigating oxidative stress. GA can also be used to manage obesity-related comorbidities including type 2 diabetes and dyslipidemia.









Similar content being viewed by others
References
Baraskar K, Thakur P, Shrivastava R, Shrivastava VK. Female obesity: Association with endocrine disruption and reproductive dysfunction. Obes Med. 2021;28(100375). https://doi.org/10.1016/j.obmed.2021.100375.
Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. Jul. 2017;23(7):804–14. https://doi.org/10.1038/nm.4350.
Maphumulo SC, Pretorius E. “Role of Circulating Microparticles in Type 2 Diabetes Mellitus: Implications for Pathological Clotting,” Semin Thromb Hemost, vol. 48, no. 2, pp. 188–205, Mar. 2022, doi: https://doi.org/10.1055/s-0041-1740150.
Khazrai YM, Defeudis G, Pozzilli P. “Effect of diet on type 2 diabetes mellitus: a review,” Diabetes Metab Res Rev, vol. 30 Suppl 1, pp. 24–33, Mar. 2014, doi: https://doi.org/10.1002/dmrr.2515.
Ota T. Obesity-Induced inflammation and insulin resistance. Front Endocrinol (Lausanne). Dec. 2014;5:204. https://doi.org/10.3389/fendo.2014.00204.
Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. Apr. 2012;19(2):81–7. https://doi.org/10.1097/MED.0b013e3283514e13.
Honma M, et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int J Obes (Lond). Sep. 2018;42(9):1544–55. https://doi.org/10.1038/s41366-018-0062-9.
Chadt A, Al-Hasani H. “Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease,” Pflugers Arch, vol. 472, no. 9, pp. 1273–1298, Sep. 2020, doi: https://doi.org/10.1007/s00424-020-02417-x.
Eckstein SS, Weigert C, Lehmann R. Divergent roles of IRS (insulin receptor substrate) 1 and 2 in liver and skeletal muscle. Curr Med Chem. 2017;24(17):1827–52. https://doi.org/10.2174/0929867324666170426142826.
Voight BF et al. “Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis,” Nat Genet, vol. 42, no. 7, pp. 579–589, Jul. 2010, doi: https://doi.org/10.1038/ng.609.
Boura-Halfon S, Zick Y. “Phosphorylation of IRS proteins, insulin action, and insulin resistance,” Am J Physiol Endocrinol Metab, vol. 296, no. 4, pp. E581-591, Apr. 2009, doi: https://doi.org/10.1152/ajpendo.90437.2008.
Lehnen A, et al. The beneficial effects of exercise in rodents are preserved after detraining: a phenomenon unrelated to GLUT4 expression. Cardiovasc Diabetol. 2010. https://doi.org/10.1186/1475-2840-9-67.
Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:2–3. https://doi.org/10.1016/j.mam.2012.07.001.
Wang T, Wang J, Hu X, Huang X-J, Chen G-X. “Current understanding of glucose transporter 4 expression and functional mechanisms,” World J Biol Chem, vol. 11, no. 3, pp. 76–98, Nov. 2020, doi: https://doi.org/10.4331/wjbc.v11.i3.76.
Choubey S, Goyal S, Varughese LR, Kumar V, Sharma AK, Beniwal V. Probing gallic acid for its broad spectrum applications. Mini Rev Med Chem. 2018;18(15):1283–93. https://doi.org/10.2174/1389557518666180330114010.
Punithavathi V, Prince PSM, Kumar R, Selvakumari J. “Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. ” Eur J Pharmacol. 2011. https://doi.org/10.1016/j.ejphar.2010.08.059.
Wang C-Y, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol. 2012;821:421–33. https://doi.org/10.1007/978-1-61779-430-8_27.
Tortoriello DV, McMinn J, Chua SC. “Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice,” Endocrinology, vol. 145, no. 3, pp. 1238–1247, Mar. 2004, doi: https://doi.org/10.1210/en.2003-1406.
Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. Apr. 2011;18(2):139–43. https://doi.org/10.1097/MED.0b013e3283444b09.
Lang P, Hasselwander S, Li H, Xia N. Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Sci Rep. 2019;9(1):19556. https://doi.org/10.1038/s41598-019-55987-x.
Hsieh Y-S, Chen P-N, Yu C-H, Chen C-H, Tsai T-T, Kuo D-Y. Involvement of oxidative stress in the regulation of NPY/CART-mediated appetite control in amphetamine-treated rats. Neurotoxicology. May 2015;48:131–41. https://doi.org/10.1016/j.neuro.2015.03.011.
Doan KV, et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology. Jan. 2015;156(1):157–68. https://doi.org/10.1210/en.2014-1354.
Cisa-Wieczorek S, Hernández-Alvarez MI. “Deregulation of Lipid Homeostasis: A Fa(c)t in the Development of Metabolic Diseases,” Cells, vol. 9, no. 12, Art. no. 12, Dec. 2020, doi: https://doi.org/10.3390/cells9122605.
Bak E-J et al. “Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice,” Scand J Clin Lab Invest, vol. 73, no. 8, pp. 607–614, Dec. 2013, doi: https://doi.org/10.3109/00365513.2013.831470.
Ma M, et al. Triglyceride is independently correlated with insulin resistance and islet beta cell function: a study in population with different glucose and lipid metabolism states. Lipids Health Dis. Jun. 2020;19(1):121. https://doi.org/10.1186/s12944-020-01303-w.
Klop B, Elte JWF, Cabezas MC. “Dyslipidemia in obesity: mechanisms and potential targets,” Nutrients, vol. 5, no. 4, pp. 1218–1240, Apr. 2013, doi: https://doi.org/10.3390/nu5041218.
Morton AM, Furtado JD, Mendivil CO, Sacks FM. “Dietary unsaturated fat increases HDL metabolic pathways involving apoE favorable to reverse cholesterol transport,” JCI Insight, vol. 4, no. 7, pp. e124620, 124620, Apr. 2019, doi: https://doi.org/10.1172/jci.insight.124620.
Tanaka M, et al. Gallic acid regulates adipocyte hypertrophy and suppresses inflammatory gene expression induced by the paracrine interaction between adipocytes and macrophages in vitro and in vivo. Nutr Res. 2020;73:58–66. https://doi.org/10.1016/j.nutres.2019.09.007.
Yoshida M et al. “High density lipoprotein inhibits the activation of sterol regulatory element-binding protein-1 in cultured cells,” FEBS Lett, vol. 584, no. 6, pp. 1217–1222, Mar. 2010, doi: https://doi.org/10.1016/j.febslet.2010.02.034.
Kron V, et al. The changes of cholesterol Profile at the different insulin resistance range in the Czech Republic. Med (Kaunas). Mar. 2021;57(3):249. https://doi.org/10.3390/medicina57030249.
Hoenig MR, Sellke FW. “Insulin resistance is associated with increased cholesterol synthesis, decreased cholesterol absorption and enhanced lipid response to statin therapy,” Atherosclerosis, vol. 211, no. 1, pp. 260–265, Jul. 2010, doi: https://doi.org/10.1016/j.atherosclerosis.2010.02.029.
Bilotta MT, Petillo S, Santoni A, Cippitelli M. Liver X receptors: regulators of cholesterol metabolism, inflammation, autoimmunity, and Cancer. Front Immunol. 2020;11:584303. https://doi.org/10.3389/fimmu.2020.584303.
Makihara H, et al. Gallic acid, the active ingredient of Terminalia bellirica, enhances adipocyte differentiation and adiponectin secretion. Biol Pharm Bull. 2016;39:1137–43. https://doi.org/10.1248/bpb.b16-00064.
Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013;4:71. https://doi.org/10.3389/fendo.2013.00071.
Shahida R, Farasat T, Naz S, Malik SJ. Expression Profile of TNF-α among impaired glucose tolerant and type 2 Diabetic subjects in relation with vascular inflammation. Pakistan J Zool. Nov. 2017;49:2153–9. https://doi.org/10.17582/journal.pjz/2017.49.6.2153.2159.
Nigro E et al. “New insight into adiponectin role in obesity and obesity-related diseases,” Biomed Res Int, vol. 2014, p. 658913, 2014, doi: https://doi.org/10.1155/2014/658913.
Obafemi TO, et al. Combined effect of metformin and gallic acid on inflammation, antioxidant status, endoplasmic reticulum (ER) stress and glucose metabolism in fructose-fed streptozotocin-induced diabetic rats. Toxicol Rep. 2021;8:1419–27. https://doi.org/10.1016/j.toxrep.2021.07.011.
Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. “ER stress-induced inflammation: does it aid or impede disease progression?,” Trends in Molecular Medicine, vol. 18, no. 10, pp. 589–598, Oct. 2012, doi: https://doi.org/10.1016/j.molmed.2012.06.010.
Paraíso AF, et al. Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue: a molecular and bioinformatic approach. Life Sci. Nov. 2019;237:116914. https://doi.org/10.1016/j.lfs.2019.116914.
Trouwborst I, Bowser SM, Goossens GH, Blaak EE. Ectopic Fat Accumulation in distinct insulin resistant phenotypes; targets for Personalized Nutritional Interventions. Front Nutr. 2018;5:77. https://doi.org/10.3389/fnut.2018.00077.
Latha R, Daisy P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. In streptozotocin-induced diabetic rats. ” Chemico-biological interactions. 2011. https://doi.org/10.1016/j.cbi.2010.11.005.
Favaretto F, et al. GLUT4 defects in adipose tissue are early signs of metabolic alterations in Alms1GT/GT, a mouse model for obesity and insulin resistance. PLoS ONE. 2014;9(10):e109540. https://doi.org/10.1371/journal.pone.0109540.
Kouidhi S et al. “Human subcutaneous adipose tissue Glut 4 mRNA expression in obesity and type 2 diabetes,” Acta Diabetol, vol. 50, no. 2, pp. 227–232, Apr. 2013, doi: https://doi.org/10.1007/s00592-011-0295-8.
Ding L, Jin D, Chen X. Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. J Nutr Biochem. Oct. 2010;21(10):941–7. https://doi.org/10.1016/j.jnutbio.2009.07.009.
Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. May 2012;13(6):383–96. https://doi.org/10.1038/nrm3351.
Leguisamo NM, et al. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc Diabetol. Aug. 2012;11:100. https://doi.org/10.1186/1475-2840-11-100.
Marques-Oliveira GH, Silva TM, Lima WG, Valadares HMS, Chaves VE. Insulin as a hormone regulator of the synthesis and release of leptin by white adipose tissue. Peptides. Aug. 2018;106:49–58. https://doi.org/10.1016/j.peptides.2018.06.007.
Gandhi GR, et al. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J Pharmacol. Dec. 2014;745:201–16. https://doi.org/10.1016/j.ejphar.2014.10.044.
Sharma M, Aggarwal S, Nayar U, Vikram NK, Misra A, Luthra K. Differential expression of insulin receptor substrate-1(IRS-1) in visceral and subcutaneous adipose depots of morbidly obese subjects undergoing bariatric surgery in a tertiary care center in north India; SNP analysis and correlation with metabolic profile. Diabetes Metab Syndr. 2021;15(3):981–6. https://doi.org/10.1016/j.dsx.2021.04.014.
Liu T et al. “A novel IRS-1-associated protein, DGKζ regulates GLUT4 translocation in 3T3-L1 adipocytes,” Sci Rep, vol. 6, no. 1, p. 35438, Dec. 2016, doi: https://doi.org/10.1038/srep35438.
Khalid M, Alkaabi J, Khan MAB, Adem A. “Insulin Signal Transduction Perturbations in Insulin Resistance,” Int J Mol Sci, vol. 22, no. 16, p. 8590, Aug. 2021, doi: https://doi.org/10.3390/ijms22168590.
Ganjifrockwala F, Joseph J, George G. Decreased total antioxidant levels and increased oxidative stress in south african type 2 diabetes mellitus patients. J Endocrinol Metabolism Diabetes South Afr. May 2017;22(2):21–5. https://doi.org/10.1080/16089677.2017.1324590.
Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. Mar. 2011;50(5):567–75. https://doi.org/10.1016/j.freeradbiomed.2010.12.006.
Mofidi Najjar F, Taghavi F, Ghadari R, Sheibani N, Moosavi-Movahedi AA. “Destructive effect of non-enzymatic glycation on catalase and remediation via curcumin,” Arch Biochem Biophys, vol. 630, pp. 81–90, Sep. 2017, doi: https://doi.org/10.1016/j.abb.2017.06.018.
Newsholme P, Keane KN, Carlessi R, Cruzat V. Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: importance to cell metabolism, function, and dysfunction. Am J Physiol Cell Physiol. Sep. 2019;317(3):C420–33. https://doi.org/10.1152/ajpcell.00141.2019.
Panic A, Stanimirovic J, Sudar-Milovanovic E, Isenovic ER. “Oxidative stress in obesity and insulin resistance,” Explor Med, vol. 3, no. 1, pp. 58–70, Feb. 2022, doi: https://doi.org/10.37349/emed.2022.00074.
Masschelin PM, Cox AR, Chernis N, Hartig SM. The impact of oxidative stress on adipose tissue Energy Balance. Front Physiol. 2019;10:1638. https://doi.org/10.3389/fphys.2019.01638.
Watson RT, Saltiel AR, Pessin JE, Kanzaki M. Subcellular compartmentalization of insulin signaling processes and GLUT4 trafficking events. In: Saltiel AR, Pessin JE, editors. ” in Mechanisms of insulin action: Medical Intelligence Unit. New York, NY: Springer; 2007. pp. 33–51. https://doi.org/10.1007/978-0-387-72204-7_2.
Acknowledgment and funding
We are thankful to the Council of Scientific &Industrial Research (CSIR), UGC, New Delhi, India for the financial support; UGC.Ref. No:445/(CSIR-UGC NET JUNE 2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of competing interest
NIL.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baraskar, K., Thakur, P., Shrivastava, R. et al. Ameliorative effects of gallic acid on GLUT-4 expression and insulin resistance in high fat diet-induced obesity animal model mice, Mus musculus. J Diabetes Metab Disord 22, 721–733 (2023). https://doi.org/10.1007/s40200-023-01194-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01194-5