Abstract
Purpose
Derangements of liver transcriptional factors and enzymes have important implications in diabetes-induced related complications. Hence, this study which consists of two experimental phases was aimed at evaluating the possible underlying molecular mechanisms of intermittent fasting (IF), exercise starvation and honey in streptozotocin (STZ)-mediated liver damage in diabetic rats.
Methods
The diabetic rats were treated orally with distilled water (0.5 ml/kg), IF, starvation and honey at 1 g/kg body weight in the non-diabetic phase for four (4) weeks. After STZ injections, four (4) weeks of IF, exercise, starvation, and honey therapy were used as interventions prior to a biochemical evaluation of the liver.
Results
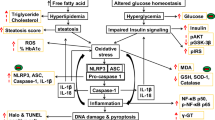
IF and exercise greatly decreased liver transcription factor (resistin, SREBP-1c), inflammatory cytokines/enzyme (TNF-α, IL-6, IL-1ß, MPO) as well as oxidative and nitrergic stress with correspondence increased liver PPAR-γ, IL-10, SOD, CAT and GSH in diabetic rats unlike starvation and honey regimen relative to diabetic controls. Furthermore, IF and exercise significantly improved hepatic glycogen synthase and decreased glycogen phosphorylase in diabetic rats compared to the diabetic control group, but starvation and honey therapy had no such influence. IF and exercise strategically reduces STZ-induced liver metabolic disorder via through modulation of liver transcriptional factors and inhibition of pro-inflammatory cytokines, oxido-nitrergic and adipokine signaling pathway.







Similar content being viewed by others
Data availability
The corresponding author can provide all of the data used in this article upon request.
Code availability
Not applicable.
Abbreviations
- STZ:
-
Streptozotocin,
- IF:
-
Intermittent fasting
- TNF-α:
-
Tumor necrotic factor-alpha,
- IL-6::
-
Interleukin-6
- IL-1β:
-
Interleukin-1 beta
- IL-10:
-
Interleukin-10
- GSH:
-
Glutathione reductase
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- MDA:
-
Malonaldehyde
- LPO:
-
Lipid peroxidation
- NO:
-
Nitric oxide,
- MPO:
-
Myeloperoxidase
- SREBP1c:
-
Sterol regulatory element binding protein 1c
- PPAR- γ:
-
Peroxisome proliferator-activated receptor-y
References
Hudish LI, Reusch JEB, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Investig. 2019;129(10):4001–8.
Erejuwa O, Nwobodo N, Akpan J, Okorie U, Ezeonu C, Ezeokpo B, Nwadike K, Erhiano E, Abdul Wahab M, Sulaiman S. Nigerian honey ameliorates hyperglycemia and dyslipidemia in Alloxan-induced diabetic rats. Nutrients. 2016;8(3):95.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–223.
Xu N, Zhang Y, Doycheva DM, Ding Y, Zhang Y, Tang J, Guo H, Zhang JH. Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPKpathway in neonatal rats. Neuropharmacology. 2018;133:415–28.
Henson J, Yates T, Edwardson CL, Khunti K, Talbot D, Gray LJ, Davies MJ. Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS ONE. 2013;8(10):78350–007835.
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–50.
Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects-a narrative review of human and animal evidence. Behav Sci (Basel, Switzerland). 2017;7(1).
Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. 2018;26(2):254–68.
Almasaudi SB, El-Shitany NA, Abbas, AT, Abdel-dayem UA, Ali SS, al Jaouni SK, Harakeh S. Antioxidant, anti-inflammatory, and antiulcer potential of Manuka Honey against gastric ulcer in rats. Oxidative Med Cell Longev. 2016;3643824.
Akanmu M, Olowookere T, Atunwa S, Ibrahim B, Lamidi O, Adams P, Ajimuda B, Adeyemo L. Neuropharmacological effects of Nigerian honey in mice. Afr J Trad Compl Alt Med. 2011;8(3).
Al Aamri ZM, Ali BH. Does honey have any salutary effect against streptozotocin - induced diabetes in rats. J Diabetes Metab Disord. 2017;16(1).
Szalai Z, Szász A, Nagy I, Puskás LG, Kupai K, Király A, ... , Varga C. Anti-inflammatory effect of recreational exercise in TNBS-induced colitis in rats: role of NOS/HO/MPO system. Oxidative Med Cell Longev. 2014.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Investig Dermatol. 1982;78(3):206–9.
Hillegass L, Griswold D, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24(4):285–95.
Van Doorn R, Leijdekkers CM, Henderson PT. Synergistic effects of phorone on the hepatotoxicity of bromobenzene and paracetamol in mice. Toxicology. 1978;11:225–33.
Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71.
Evans JL, Maddux B, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7(7–8):1040–52.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–6.
Whitton PD, Hems DA. Glycogen synthesis in the perfused liver of streptozotocin-diabetic rats. Biochem J. 1975;150(2):153–65.
Rao PV, Pugazhenthi S, Khandelwal RL. The effects of streptozotocin-induced diabetes and insulin supplementation on expression of the glycogen phosphorylase gene in rat liver. J Biol Chem. 1995;270(42):24955–60.
Solini A, Di Virgilio F, Chiozzi P, Fioretto P, Passaro A, Fellin R. A defect in glycogen synthesis characterizes insulin resistance in hypertensive patients with type2 diabetes. Hypertension. 2001;37(6):1492–6.
Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52(8):1686–8.
Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by O-linked N-acetylglucosamine. J Biol Chem. 2003;278:10022–7.
Bollen M, Keppens S, Stalmans W. Specific features of glycogen metabolism in the liver. Biochem J. 1988;336(1):19–31.
Abdulwahab DA, El-Missiry MA, Shabana S, Othman AI, Amer ME. Melatonin protects the heart and pancreas by improving glucose homeostasis, oxidative stress, inflammation and apoptosis in T2DM-induced rats. Heliyon. 2021;7(3):06474.
Griendling KK, FitzGerald GA. Oxidative Stress and Cardiovascular Injury. Circulation. 2003;108(16):1912–6.
Yubero-Serrano, EM, Delgado-Lista J, Peña-Orihuela P, Perez-Martinez P, Fuentes F, Marin C, Tunez I, Jose Tinahones F, Perez-Jimenez F, Roche HM, Lopez-Miranda J. Oxidative stress is associated with the number of components of. 2013
El-Missiry MA, Amer MA, Hemieda FA, Othman AI, Sakr DA, Abdulhadi HL. Cardioameliorative effect of punicalagin against streptozotocin-induced apoptosis, redox imbalance, metabolic changes and inflammation. Egypt J Basic Appl Sci. 2015;2(4):247–60.
Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complicat. 2012;26(6):483–90.
Kogel V, Trinh S, Gasterich N, Beyer C, Seitz J. Long-term glucose starvation induces inflammatory responses and phenotype switch in primary cortical rat astrocytes. J Mol Neurosci. 2021;71(11):2368–82.
Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113(4):389–98.
Zhang B, Shen Q, Chen Y, Pan R, Kuang S, Liu G, Sun G, Sun X. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci Rep. 2017;7(1).
ALmohaimeed HM, Mohammedsaleh ZM, Batawi AH, Balgoon MJ, Ramadan OI, Baz HA, al Jaouni S, Ayuob NN. Synergistic anti-inflammatory and neuroprotective effects of Cinnamomum cassia and Zingiber officinale Alleviate diabetes-induced hippocampal changes in male Albino rats: structural and molecular evidence. Front Cell Dev Biol. 2021;9.
Bastard JP, Maachi M, van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87(5):2084–9.
Dinarello CA. Blocking interleukin-1β in acute and chronic autoinflammatory diseases. J Intern Med. 2010;269(1):16–28.
Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114(3):525–31.
Jayaraman S, Devarajan N, Rajagopal P, Babu S, Ganesan SK, Veeraraghavan VP, Palanisamy CP, Cui B, Periyasamy V, Chandrasekar K. Sitosterol circumvents obesity induced inflammation and insulin resistance by down-regulating IKK_/NF-_B and JNK signaling pathway in adipocytes of Type 2 diabetic rats. Molecules. 2021;26:2101.
Kheiripour N, Karimi J, Khodadadi I, Tavilani H, Goodarzi MT, Hashemnia M. Silymarin prevents lipid accumulation in the liver of rats with type 2 diabetes via sirtuin1 and SREBP-1c. J Basic Clin Physiol Pharmacol. 2018;29(3):301–8.
Jumli MN, Nadeem MI. The Mechanistic and Pathophysiological Role of Adiponectin and Resistin towards Regulation of Food Intake and Appetite in Cardiovascular Associated Risk Factor of Metabolic Syndrome, Type 2 Diabetes - From Pathophysiology to Cyber Systems. Anca Pantea Stoian, IntechOpen, 2021.
Acknowledgements
The authors thank the technical staff at the Department of Physiology at Delta State University in Abraka, Nigeria.
Author information
Authors and Affiliations
Contributions
Conceptualization E.A.C., N.E.K; data curation, writing original draft preparation. E.A.E., O.M.O., BBA B.O.O., EGM; review and editing BBA, O.M.O., E.G.M., N.E.K and E.V.; supervision.N.E.K.; validation N.E.K.; funding acquisition E.A.C., B.O.O and O.M.O. All authors have read and agreed to the publishing of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The Ethical Review Committee of Delta State University gave their approval to perform this study on 09/11/2021, with the reference number REC/FBMS/DELSU/21/121.
Consent to participate
Not applicable.
Consent for publication
All authors gave their consents for the article to be published.
Conflicts of interest
There were no conflicts of interest decleared by the authors.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agbonifo-Chijiokwu, E., Nwangwa, K.E., Oyovwi, M.O. et al. Underlying biochemical effects of intermittent fasting, exercise and honey on streptozotocin-induced liver damage in rats. J Diabetes Metab Disord 22, 515–527 (2023). https://doi.org/10.1007/s40200-022-01173-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01173-2