Abstract
Background
The oxidative stress caused by the creation and breakdown of reactive oxygen species affects glucose tolerance, B-cell function, insulin resistance, and metabolites containing free fatty acids. Functioning foods are therefore becoming increasingly popular because they provide health benefits and prevent oxidative stress. This research aims to assess strategies to alleviate oxidative stress and inflammation in patients with type 2 diabetes (T2DM). In the present study, the metabolic effect wheat bread fortified with pomegranate peel powder(PPP) will be assessed in participants with type 2 diabetes.
Methods
A randomized, triple-blind, placebo-controlled, and parallel arms clinical trial will be conducted on 90 patients with T2DM. Run-in courses will last for two weeks. The intervention and control groups will receive wheat bread with and without PPP, respectively. Anthropometric data, fasting plasma glucose, hemoglobin A1C, lipid profile, insulin level, high-sensitivity C-reactive protein (hs-CRP), malondialdehyde (MDA), Total antioxidant capacity(TAC), and mood state, will be measured at the baseline and three months post-intervention. Beta-cell function (HOMA-B) and insulin resistance (HOMA-IR) will also be assessed.
Discussion
This trial will provide novel data on the impact of fortified bread with PPP on metabolic profile and mood state of patients with type 2 diabetes. The results will demonstrate the potential of such intervention in glycemic indices, antioxidant status, inflammation and mood in these patients.
Trial Registration
Trial is registered in the Iranian Registry of Clinical Trials (ID: IRCT20191209045672N1). Date of registration 21/09/2020. https://en.irct.ir/trial/48132.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background and rationale
Type 2 diabetes mellitus (T2DM) is a growing global health issue [1]. The disease imposes high healthcare costs and deteriorates the patients’ quality of life [2]. The International Diabetes Federation estimates that there are currently 425 million diabetics worldwide and the number will increase to 629 million by 2045 [3]. Diabetes and insulin resistance are associated with oxidative stress, a pathogenic mechanism [4], which elevates the risk of T2DM complications including nephropathy, neuropathy, retinopathy, and accelerated coronary artery disease [5]. Researches have demonstrated that high glucose levels by firing reactive oxygen species(ROS) result in B-cell damages and lead to impaired insulin release and insulin resistance. The antioxidant protect system is accountable for the neutralization of ROS.
T2DM is currently managed by lifestyle modification and increased physical inactivity. Drug therapy becomes necessary over time to ensure reasonable glycemic control [6]. Medical nutrition therapy has been recommended as an effective method of controlling blood glucose levels in patients with T2DM avoiding the side effects of medication [7]. Considering the confirmed benefits of functional food components, these compounds can be a part of public health diet behaviors [8]. Functional food, apart from its main nutritional functions, provides significant physiological and healthy benefits [9].
PPP is discussed as a functional food and a nutritious by-product.
Based on previous systematic reviews, PPP consumption has a positive association with chronic disease prevention [10]. PPP possesses anti-inflammatory, hypoglycemic, anti-apoptotic, and prebiotic properties [11]. Studies have demonstrated that adherence to pomegranate peel extract reduces the risk of systolic blood pressure, hs-CRP, and Total cholesterol serum levels [12]. This is in line with the Rafraf study, in which intake of (500 mg) twice daily for two months was associated with decreased Cholesterol, TG, MDA, and TAC levels [12].
PPP consists of an internal network of membranes that makes up almost 30% of the total weight of the fruit. It contains not only fiber, also, ellagic acid, gallic acid and punicalagin. In addition to their free radical-scavenging properties. Also, it possess antibacterial activities against intestinal flora, particularly enteric pathogens, i.e., Escherichia coli, Shigellaspp., Salmonella spp and Vibrio cholerae [10].
Objectives
Since bread is an essential part of the most communities diet [13, 14], its fortification can play a significant role in reversing the nutritional deficiencies at the national level. Previous studies mainly assessed the effects of fortified bread with PPP in animal studies and on rheological indices in experimental circumstances [15, 16]. In this study, we will investigate the characteristics of bread with and without PPP, as well as its effect on glycemic indices, lipid profiles, inflammation, oxidative stress biomarkers, and mood status in type2 diabetes patients.
Study aims
General aim
The current study aims to evaluate the quality and antioxidant properties of bread fortified with PPP and to compare the metabolic effects of the 12-week consumption of fortified and plain wheat breads in patients with T2DM.
The specific objectives and hypotheses
-
Aim 1: To determine the glycemic effect of some breads by measuring circulating levels of pre- and post-prandial blood sugar.
-
Aim2: To determine the properties of PPP-fortified bread by sensory method and selecting the appropriate PPP percentage.
-
Aim 3: To determine the number of polyphenols in bread fortified with PPP and wheat bread.
-
Aim 4: To analyze the antioxidant capacity of bread fortified with PPP and wheat bread using the 2, 2–1-diphenyl-1-picrylhydrazyl (DPPH) assay.
-
Aim 5: To determine the anthropometric indices in the intervention and control groups pre- and post-the intervention. We hypothesize that PPP should reduce body mass index (BMI) and waist circumference.
-
Aim 6: To determine whether PPP bread improves Fasting blood glucose(FBS), HbA1c levels and insulin resistance (IR).
-
Aim 7: To determine whether PPP bread decrease the lipid profile, i.e., serum triglyceride (TG), high-density lipoprotein-C (HDL-C), and low-density lipoprotein (LDL), of patients with T2DM.
-
Aim 8: To determine mean levels of malondialdehyde (MDA) in the intervention and control groups pre- and post- the intervention. We hypothesize that PPP-fortified wheat bread will reduce serum MDA in these individuals.
-
Aim 9: To determine the total antioxidant capacity (TAC) in the intervention and control groups pre- and post- the intervention. According to our hypothesis, PPP-fortified wheat bread will improve TAC in individuals with T2DM.
-
Aim 10: To determine hs-CRP levels in the intervention and control groups pre- and post- the intervention.
-
Aim 11: To determine whether PPP-fortified wheat bread improves mood state (stress, anxiety, and depression scores).
We hypothesize that PPP consumption improves glycemia, beta-cell function, quality of life, inflammation markers and reduces oxidative stress.
Material and methods
Experimental protocol
The current research will be conducted in three phases:
-
Phase one: To explore the postprandial effects of five wheat bread products on patients with T2DM.
-
Phase two: To evaluate the organoleptic characteristics of bread prepared with four doses of PPP and measure total phenol, DPPH and MTT colorimetric assay.
-
Phase three: To design a parallel-group randomized triple-blind clinical trial for assessing the bread fortification effects on metabolic indices of patients with T2DM.
Phase one
Dough preparation and baking procedure
In this study, flat and semi-bulky breads are used. Wheat flour, rye, salt, sugar, and yeast are mixed during bread making. A mature sourdough starter was used to make bread. The baking time for flatbreads will be approximately 3 min at a temperature is almost 500 °C, while the baking time for semi-bulky breads was 20 min at 210 °C.
Phase two
Pomegranate peel powder preparation
Pomegranates of a specific variety will be collected and from cultivation areas in Neyriz, Fars Province, southwest of Iran. They will be washed with cold water and drained. They will be then cut open and the leathery skin on the outside will be removed. Then, the pomegranate and white parts of the interior pomegranate will be separated. The skin will be ground using an electric mill and sieved through a #12 mesh sieve. The powder will be stored in closed containers in a -20 °C freezer further until use.
Chemical composition of bread with and without PPP
Measurements will be conducted on bread with and without PPP in terms of protein, carbohydrate, fat, and fiber. Protein, Moisture, fat, fiber and carbohydrates (by difference) in the breads be determined by the methods of AOAC [17].
Organoleptic (sensory) tests
Different amounts of PPP will be added to wheat flour to obtain final percentages of 1.5%, 2.5%, 3.5%, and 5%. PPP-free samples will also be used as controls. The prepared flat and semi-bulky breads will be cut into equal-sized slices and served in unmarked dishes for 25 untrained panelists (university employees and students) to score their organoleptic properties on a five-point hedonic scale from one (extremely poor or lowest quality) to five (remarkably good or excellent quality). They will be asking to drink some water after testing each sample to cleanse their palate. We will also record and rate the internal and external characteristics of texture, color, aroma, taste, and mouth feel of the bread samples on a scored sheet.
Determination of total phenol
Folin-Ciocalteu (FC) colorimetric method will be employed to determine the total phenolic content (TPC) of methanolic extracts of bread samples. Results will be expressed in milligrams of Gallic acid equivalents per gram of bread (mg GAE/g) [11, 15].
DPPH radical scavenging activity
DPPH will be used to assess the antioxidant activity of the extract. Using a spectrophotometer, the absorbance is measured at 515 nm, and the percentage of DPPH scavenging activity will be calculated using the following equation [16]:
The experiment will be performed three times and the average value will be reported. The amount of radical scavenging activity will then be calculated. Half-maximal inhibitory concentration (IC50) will be used to determine the substance’s ability to neutralize 50% of the initial free radicals in the environment. Phenolic and DPPH tests will be conducted to determine the phenol content of bread after adding PPP.
MTT test
As a rapid and sensitive method for assessing materials cytotoxicity, the MTT test will be widely used. A total of 10,000 cells will be added to each well of the 96-well plates, along with 180 µl of RPMI culture medium. Then, various dilutions of pomegranate peel extract will be added to the wells. In the control group, no extract will be added to the cells, and only water will be added. In addition, doxorubicin and DMSO (1%) will be used as positive and negative controls, respectively. Under the same conditions, the plate is incubated for 24 h. 100 ml of MTT solution was added to each well and set for 3 h at 37 °C to assess cell viability. After removing the old contents containing MTT, 100 µl of DMSO will be pipetted into each well to dissolve the formazan crystals. At 570 nm, the absorbance will be measured with an ELISA reader (Startfix-2100, Awareness, USA). Modelling the percentage of cytotoxicity against extracts gives the concentrations that inhibit half of the cell population (IC50). The following formula will be used to calculate the rate of alive cells. The assay will done in triplicate and results were reported as mean ± standard deviation (SD). The cell viability (%) was determined according to below equation [18].
where b = blank, and c = control.
Phase three
Setting
Initially, a list of outpatients with T2DM will be obtained from the University Endocrine and Metabolism Research Center as well as other health centers in Isfahan, Iran.
Eligibility criteria
Study inclusion and exclusion criteria are in Table 1.
Study participants
Volunteer patients will be invited to contact the research team by phone, attend an examination, and complete a brief questionnaire about their health history. The study protocol will be explained to the participants and they will be asked not to make any changes to their usual diet during the study. Groups 1 and 2 will receive wheat bread (100 g) named A and B, respectively. Both the researchers and the patients will be blinded to the randomization code of the intervention and placebo bread (A and B).
Informed consent form process
Informed consent will be obtained from all participants. An approval from the Medical Ethics Committee of Isfahan University of Medical Sciences will also be needed. Before their enrollment, the patients will be assured that their unwillingness to participate will not affect their usual health care at health centers. The participants will have enough time to review the consent form and ask any questions they should have. They will also receive a copy of the consent document after signing it.
Sample size calculation
The sample size is calculated by considering the first type error (α = 0.05), the second type error (β = 0.2), SD = 32 and 42, and the minimum significant difference for cholesterol (23 mg/dl) as the main outcome variable [12].
The sample size for each group is 40 participants. By adding a 10% chance of dropping out, 45 people will be yielded. Sampling is expected to last for 14 months. The participants’ data will be recorded even if they withdraw from the study [12].
Run-in period
The study will last for three months, beginning with a 14-day run-in period. The participants will be asked to maintain their dietary habits, medicines, and exercise. The run-in period will help the researchers to become familiar with the patients’ diets and to identify their concerns. During the run-in period, the participants will be consuming wheat bread without PPP. The run-in phase is necessary to test the volunteers’ motivation to comply with the intervention. Since there is no biomarker to measure bread intake, we will use diaries to assess their compliance.
Study design and intervention
This article follows the SPIRIT (Standard Protocol Items: Recommendations for International Trials) guidelines for reporting clinical trial protocols [17].
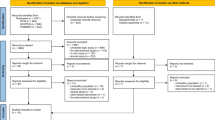
The trial is a randomized triple-blind, placebo-controlled clinical trial with a parallel-group design. The study will be conducted on 90 diabetic patients referred to Univercity Endocrine and Metabolism Research Center and other health centers in the city. Diabetic patients are defined according to ADA criteria [19]. Patients will be briefed with the reasons for the work and how the plan is implemented by telephone and face to face contact. A questionnaire containing demographic data, age, gender, telephone number, cell phone, address, and medical records will be used to evaluate the inclusion criteria. Individuals who wish to participate in the study will be asked to sign an informed consent form after the approval of the study. The participants will be assigned to either the intervention or control group (Fig. 1) and both groups will continue their routine treatment plans. In addition to their usual diet, the participant will receive a packet of 100 gr of bread. Participants will be given a packet of bread for one week and visited weekly to assess their compliance and provide them with bread packet for the next one week. Also, participant’s compliance with the consumption of bread will be monitored through telephone interviews once a week. The participants will simply randomize to one of two treatments: a packet of bread per week containing placebo (without PPP) and intervention (with PPP). Table 2 displays the enrollment, interventions, assessments, and participants’ visits. If participants change their medicine dose or any problems arise, they will be excluded from the study.
Anthropometric parameters
We will measure height, weight, and waist and hip circumferences at the baseline and three months’ post-intervention. Height and weight (with a precision of 0.01 kg) will be measured using a scale-mounted stadiometer while the patients wearing light clothing and no shoes. Each participant’s BMI will be calculated as the result of dividing their weight (in kg) by their height (in m) squared. A non-flexible tape will be used to measure waist and hip circumference to the nearest 0.1 cm. Waist circumference(WC) will be calculated at the midpoint between the lowest rib and the iliac crest while the patient’s body is level and wearing minimal clothing. Hip circumference will be measured around the widest portion of the buttocks. Only one person will do measurements to reduce the errors.
Physical activity and dietary intake evaluation
At the beginning of the intervention and 12 weeks later, food and activity records will be assessed. A trained nutritionist will explain how to fill out the food and physical activity record. For dietary intake evaluation, the participants will be asked to record their daily food and beverage intake for three days. We will assess dietary intake using the food records of three non-consecutive days (two weekdays and a weekend). A food scale and a model will also be used to improve accuracy. The three-day food records will be reviewed by the investigator in the presence of the patient. We will convert the portion sizes to grams. Using a customized Nutritionist 4 software (First data bank Inc., Hearst Corp., San Bruno, CA), each food and beverage item will be coded and analyzed for energy content and macro- and micronutrients. Individuals consuming less than 1200 kcal/day or more than 4000 kcal/day will be excluded. The Participants will complete the Metabolic Equivalent Questionnaire (MET) for physical activity pre- and post- the intervention and will be asked to report their physical activity daily.
Blood pressure
Blood pressure will be measured after 10 min of resting in a seated position using a mercury barometer calibrated in that position. The patients will be asked not to drink tea/coffee or engage in heavy physical activity for approximately 30 min before the measurement. At the beginning and end of the study, blood pressure measurements will be performed twice and the mean of two consecutive readings will be recorded.
Biochemical assays
To do laboratory tests, patients who meet the inclusion criteria (Table 1) will be asked to attend University Endocrine and Metabolism Research Center between 8:00 am and 10:00 am after fasting for 12 h. During their appointment, ten mL blood samples will be obtained. The collected samples will be centrifuged at 3500 rpm, 25 ℃ for 15 min. The serum will then be transferred and stored in a -80 ℃ freezer until further analysis. The participants will be tested for their biochemical parameters at the baseline and post- the intervention. FBS levels will be assessed using the glucose oxidase method. Inter- and intra-assay coefficients of variation are ≤ 2% for glucose. Glycated hemoglobin (HbA1c) will be determined by turbidimetric method. Serum insulin will be assessed by enzyme-linked immunosorbent assay (ELISA) (Monobind Inc, Costa Mesa, CA, USA). The homeostatic model assessment of insulin resistance (HOMA-IR) and homeostatic model assessment of beta-cell function (HOMA-B) index will be used to determine insulin resistance and beta-cell function, respectively. To assess insulin sensitivity, the check index (Quicki) will be measured based on the following equation:
HOMA-IR = (glucose-insulin)/405; Where glucose is fasting glucose (mg/dL) and insulin is fasting insulin (mu/mL) [18, 20, 21]. Plasma lipid and lipoprotein concentrations (i.e., total cholesterol, high- and low-density lipoprotein cholesterol, and triglycerides) will be measured using a photometric assay kit (Dialab, Austria). Inter- and intra-assay coefficients of variation are ≤ 2% for triacylglycerols and cholesterol. Serum high sensitivity C-reactive protein (hs-CRP) levels will be assessed by the turbidimetric immunoassay method (APTEC Diagnostics NV, Belgium). Inter- and intra-assay coefficients of variation are ≤ 3%.
Malondialdialhyde (MDA) concentration will evaluation based on thiobarbituric acid reactive substance using commercial kit (kiazist, Iran). Total antioxidant capacity (TAC) will be assessed based on the ferric reducing antioxidant power method using a commercial kit (kiazist, Iran).
Intra-assay and interassay coefficients of variation for MDA and TAC are less than 10%. Depression, Anxiety, Stress scale-21(DASS-21) will use to assess the state mood of the participants. DASS-21 is a self-report questionnaire with three subscales that contains seven questions related to each subscale [19, 22].
Randomization, allocation concealment, and blinding
Random allocation software generates a series of sequentially numbered envelopes containing equal assignments of wheat bread without or with PPP. An independent researcher who is not involved in the design or the analysis of the study, will label the breads and assigned them to participant ID according to the randomization sequence output to ensure that the survey will be triple-blinded to researchers, participants and statistitian.
Data management
Access to the server is password protected, as is key to the research database. All collected data will be treated confidential at all times will only be used anonymus for all assessment. The data from the 3-d dietary records will be entered to SPSS by investigators and double checked.
Statistical analyses
Results and adverse events will be double-checked using SPSS software (Version 20). It is validated by SPSS Inc., Chicago, IL, USA. Missing data, outliers, and violations of normality will be assessed.
For continuous variables, data will be expressed as mean ± SD, while for qualitative variables, a frequency report will be provided. To determine whether the data is under the normal distribution, we will use the Kolmogorov–Smirnov test. Paired samples t-test will be used to compare the before and after values within each study group. Independent samples t-test will be used to compare baseline and after data between the two study groups. Mann-Withney test for non-normal data will be applied. The mean of differences between baseline and 3 months in primary and secondary outcomes will be calculated for each of the groups and will be compared by independent samples t-test. Univariate analysis of covariance (ANCOVA) will be used to control for confounders including baseline values. Since these parameters are not normally distributed, non-parametric tests will be used. Mann-Withney or Wilcoxon signed-rank tests will be used as appropriate to check for changes within and between groups.
Two-sided tests will be used. Also, in all analyses, the value of P-value < 0.05 will be considered statistically significant.
Patient and public involvement
Information is certainly disseminated to the public especially diabetic patients via any media coverage of study findings.
Study outcomes
Primary outcomes
The primary outcomes of this clinical trial are the changes in HbA1C, FBS, insulin, lipid profile (triglyceride, total cholesterol, HDL-cholesterol, LDL-cholesterol), Oxidative stress biomarkers such as TAC, inflammation factors such as MDA and mood states at the end of the study in comparison with the baseline values.
Secondary outcomes
The secondary outcomes of this clinical trial are weight changes, BMI, WC and blood pressure.
Monitoring and follow-up period
Adverse events, enrollment numbers, loss to follow up counts, and withdrawals will be monitored by the research team.
The research team will check the bread consumption every week.
In addition, if patients would have any complications during the intervention, they will be asked to stop the trial, and the endocrinologist will manage the required treatment.
Strengths and limitations
-
While previous studies have assessed the health effects and organoleptic characteristics of PPP, we will use this additive in a clinical trial.
-
Appropriate measures will be adopted to determine the best percentage of PPP to improve the rheological, organoleptic, and antioxidant characteristics of wheat bread.
-
Effects of bread intervention on diabetes profile, antioxidant capacity, inflammatory markers, and mood disorders in patients with T2DM will also be assessed.
-
PPP-fortified bread will be applied in a trial to show whether it can reduce signs associated with diabetes, hyperlipidemia, and oxidative stress as a functional food.
-
All participants will be obliged to maintain a stable pattern of physical activity and diet.
-
Compliance could decline over the 12-week follow-up.
Discussion
Through our research, we will seek to determine the efficacy and safety of PPP for T2DM. The Iranian meal is incomplete without bread. In many countries, bread is economically accessible to everyone. Increasing consumer demand for functional foods opens up a massive market for this category. Importantly, our findings could have a significant impact on clinical practice and public health as a simple and effective adjunctive approach for management of T2DM if our hypotheses are supported.
Trial status
The recruitment of participants began in May 2021 and was expected to be completed in October 2022. Due to the Covid-19 pandemic, we have had a lot of drop-outs, and hence, the study took longer than expected. The intervention and follow-ups are scheduled to be completed in January 2023.
Data availability
The data that supports the findings of this study will be available from the corresponding author upon reasonable request.
References
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1(1):1–22.
Guerrero-Fernández de Alba I, et al. Comorbidity in an older population with type-2 diabetes mellitus: identification of the characteristics and healthcare utilization of high-cost patients." Front Pharmacol. 2020;11:586187.
Mellitus D. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43.
Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biomed J. 2017;40(5):257–62.
Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12(1):5.
McGavock J, Dart A, Wicklow B. Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr DiabRep. 2015;15(1):568.
Early KB, Stanley K. Position of the Academy of Nutrition and Dietetics: the role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J Acad Nutr Diet. 2018;118(2):343–53.
Moreira-Rosário A, et al. Can wheat germ have a beneficial effect on human health? A study protocol for a randomized crossover controlled trial to evaluate its health effects. BMJ Open. 2016;6(11):e013098.
Viuda-Martos M, et al. Role of fiber in cardiovascular diseases: a review. Compr Rev Food Sci Food Saf. 2010;9(2):240–58.
Ismail T, Sestili P, Akhtar S. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol. 2012;143(2):397–405.
Ismail T, Akhtar S, Riaz M, Ismail A. Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies. Int J Food Sci Nutr. 2014;65(6):661–6.
Haghighian MK, Rafraf M, Moghaddam A, Hemmati S, Jafarabadi MA, Gargari BP. Pomegranate (Punica granatum L.) peel hydro alcoholic extract ameliorates cardiovascular risk factors in obese women with dyslipidemia: A double blind, randomized, placebo controlled pilot study. Eur J Integr Med. 2016;8(5):676–82.
Ohimain EI. Recent advances in the production of partially substituted wheat and wheatless bread. Eur Food Res Technol. 2015;240(2):257–71.
Tangestani H, Djafarian K, Shab-Bidar S. The effect of daily consumption of different doses of fortified Lavash bread versus plain bread on serum vitamin-D status, body composition, metabolic and inflammatory biomarkers, and gut microbiota in apparently healthy adult: study protocol of a randomized clinical trial. Trials. 2019;20(1):1–8.
Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96(2):254–60.
Chen X, Liang L, Han C. Borate suppresses the scavenging activity of gallic acid and plant polyphenol extracts on DPPH radical: A potential interference to DPPH assay. LWT. 2020;131: 109769.
Chan AW, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Ostrowska L, Witczak K, Adamska E. Effect of nutrition and atherogenic index on the occurrence and intensity of insulin resistance. Pol Arch Med Wewn. 2013;123(6):289–96.
Sahebi A, Asghari MJ, Salari RS. Validation of depression anxiety and stress scale (DASS-21) for an Iranian population. 2005. p. 36–54.
Hřebíček JI, Janout VR, Malinčíková J, Horáková D, Čížek LK. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metabol. 2002;87(1):144.
Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, Gude F, Cadarso-Suarez C, García F, et al. Insulin resistance index (HOMA-IR) levels in a general adult population: curves percentile by gender and age. The EPIRCE study. Diabetes Res Clin Pract. 2011;94(1):146–55.
Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44(2):227–39.
Acknowledgements
The authors wish to thank the staff of University Endocrine and Metabolism Research Center and Ms. Azam Khani , Marzieh Najafi and Dr. Nazila Kassaian, Dr Sahar Saraf-Bank, Dr Mohammad Sadegh Damavandi and Dr Azra Salehi for their assistance in this study. We would also like to thank Simin Bread Company for providing the bread. Furthermore, the investigators are grateful to the patients participating in this project for their commitment and time.
Funding
This project was financially supported by the Isfahan University of Medical Sciences (grant number 398978). The sponsor have no role in study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submitting the information for publication.
Author information
Authors and Affiliations
Contributions
Reza Amani and Maryam Zare designed the initial idea of this study, the statistical analysis plan, carrying out the trial, writing protocol manuscript and final revision. RA supervised the study.
Amir Hossein Goli participated in study design and final revision.
Mozhgan Karimifar managed the patients as an endocrinologist, and participated in final revision.
Mohammad Javad Tarrahi helped with, desiging the protocol statistical analysis.
Atefe Rezaei helped with writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Individuals will provided written informed consent the study will be conducted in compliance with the Declaration of Helsinki: The trial has received ethical approval from the Ethics committee of Isfahan University of Medical Sciences (number: IR.MUI.RESEARCH.REC.1399.087). The study results will be disseminated through peer-reviewed publications and presentations at scientific meetings.
Patient and public involvement
No patient or public involvement.
Multi-publication
Because this study examines many variables, many articles will be extracted from it.
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zare, M., Goli, A.H., Karimifar, M. et al. Effect of bread fortification with pomegranate peel powder on glycemic indicators, antioxidant status, inflammation and mood in patients with type 2 diabetes: study protocol for a randomized, triple-blind, and placebo-controlled trial. J Diabetes Metab Disord 22, 921–929 (2023). https://doi.org/10.1007/s40200-022-01168-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01168-z