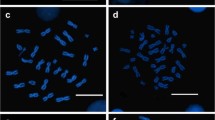
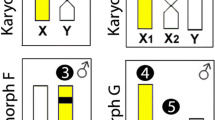
Abstract
Purpose of Review
Fishes exhibit the greatest biodiversity among extant vertebrates. In fact, about 34,000 fish species are currently estimated, of which ~ 25% are living in Neotropical freshwaters. Currently, several leading-edge studies using molecular biology procedures have largely contributed to the investigation of the fish genomic architecture at the chromosomal level. In this review, we intend to demonstrate that conventional cytogenetics is also a powerful procedure to identify and clarify both individual and inter- or intrapopulational fish characteristics and to unveil their biodiversity.
Recent Findings
Intra- or interpopulational chromosomal characteristics, revealing dramatic processes of evolution and cryptic divergence and even speciation, as well as unusual cases of interspecific hybridization, clonal reproduction, and sex chromosome differentiation, were, and still are, unmistakably discovered among fishes by using conventional, i.e., non-molecular cytogenetic procedures.
Summary
In this review, we aim to demonstrate that conventional cytogenetics constitutes a powerful and indispensable tool in characterizing the hidden biodiversity of the ichthyofauna. We focus on some key examples that clearly illustrate the importance and the efficiency of this approach.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
•• Nelson JS, Grande TC, Wilson MVH. Fishes of the World. John Wiley & Sons; 2016. This book is a reference for fish classification, systematics, and distribution.
Hoffmann AA, Rieseberg LH. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annu Rev Ecol Evol Syst. 2008;39:21–42.
•• Wellenreuther M, Bernatchez L. Eco-evolutionary genomics of chromosomal inversions. Trends Ecol Evol. 2018;33:427–40 This paper describes important roles of inversions in the evolution and adaptation.
Ravi V, Venkatesh B. Rapidly evolving fish genomes and teleost diversity. Curr Opin Genet Dev. 2008;18:544–50.
Ráb P, Bohlen J, Rábová M, Flajšhans M, Kalous L. Cytogenetics as a tool box in fish conservation: the present situation in Europe. In: Fish Cytogenet. Enfield NH, USA: Science Publishers; 2006.
• Yano CF, LAC B, Ezaz T, Trifonov V, Sember A, Liehr T, et al. Highly conserved Z and molecularly diverged W chromosomes in the fish genus Triportheus (Characiformes, Triportheidae). Heredity. 2017;118:276–83 This paper documents otherwise rarely observed monophyletic origin of ZZ/ZW sex chromosome system as well as different degree of W differentiation within a single fish genus.
Ferguson-Smith MA. History and evolution of cytogenetics. Mol Cytogenet BioMed Central. 2015;8:19.
Puddington MM, Muzio RN. Relación entre conducta y activación de áreas cerebrales. Empleo de la técnica de AgNOR en psicología comparada. Interdisciplinaria. 2016;33:129–41.
Perazzo GX, Noleto RB, Vicari MR, Gava A, Cestari MM. B chromosome polymorphism in south American cichlid. Neotrop Biodivers. 2018;4:3–9.
Cioffi MB, Molina WF, Artoni RF, Bertollo LAC. Chromosomes as tools for discovering biodiversity. The case of Erythrinidae fish family BT - recent trends in cytogenetic studies. Methodologies and applications. In: Tirunilai P, editor. Rijeka: Intech; 2012.
Ráb P, Slavík O. Diploid-triploid-tetraploid complex of the spined loach, genus Cobitis in Psovka Creek: the first evidence of the new species of Cobitis in the ichthyofauna of the Czech Republic. Acta Univ Carol Biol. 1996;39:201–14.
Bertollo LAC, de Bello Cioffi M, Galetti PM Jr, Moreira Filho O. Contributions to the cytogenetics of the Neotropical fish fauna. Comp Cytogenet. 2017;11:665–90.
•• Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol. 2016;89:12–47 This paper describes general aspects of fish distribution in South American region.
Eschmeyer WN, Fricke R, van der Laan R. Catalog of fishes: genera, species, references. [Internet]. 2018 [cited 2018 Jun 15]. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
Ferreira M, Kavalco KF, de Almeida-Toledo LF, Garcia C. Cryptic diversity between two Imparfinis species (Siluriformes, Heptapteridae) by cytogenetic analysis and DNA barcoding. Zebrafish. 2014;11:306–17.
Ramirez JL, Birindelli JL, Carvalho DC, Affonso PRAM, Venere PC, Ortega H, et al. Revealing hidden diversity of the underestimated Neotropical ichthyofauna: DNA barcoding in the recently described genus Megaleporinus (Characiformes: Anostomidae). Front Genet. 2017;8:149.
Ferreira-Neto M, Artoni R, Artoni R, Vicari M, Moreira Filho O, Camacho J, et al. Three sympatric karyomorphs in the fish Astyanax fasciatus (Teleostei, Characidae) do not seem to hybridize in natural populations. Comp Cytogenet. 2012;6:29–40.
Ferreira M, Garcia C, Matoso DA, de Jesus IS, Cioffi MB, Bertollo LAC, et al. The Bunocephalus coracoideus species complex (Siluriformes, Aspredinidae). Signs of a speciation process through chromosomal, genetic and ecological diversity. Front Genet. 2017;8:120.
Kavalco KF, Brandão K de O, Pazza R, de Almeida-Toledo LF. Astyanax hastatus Myers, 1928 (Teleostei, Characidae): a new species complex within the genus Astyanax? Genet Mol Biol. 2009;32:477–83.
Milhomem SSR, Pieczarka JC, Crampton WGR, Silva DS, De Souza ACP, Carvalho JR, et al. Chromosomal evidence for a putative cryptic species in the Gymnotus carapo species-complex (Gymnotiformes, Gymnotidae). BMC Genet. 2008;9:75.
Nakayama C, Jégu M, Porto JIR, Feldberg E. Karyological evidence for a cryptic species of piranha within Serrasalmus rhombeus (Characidae, Serrasalminae) in the Amazon. Copeia. 2001;2001:866–9.
do Nascimento VD, Coelho KA, Nogaroto V, de Almeida RB, Ziemniczak K, Centofante L, et al. Do multiple karyomorphs and population genetics of freshwater darter characines (Apareiodon affinis) indicate chromosomal speciation? Zool Anz. 2018;272:93–103.
Nirchio M, Paim FG, Milana V, Rossi AR, Oliveira C. Identification of a new mullet species complex based on an integrative molecular and cytogenetic investigation of Mugil hospes (Mugilidae: Mugiliformes). Front Genet. 2018;9:1–9.
Oliveira IA, Argolo LA, Bitencourt J de A, Diniz D, Vicari MR, Affonso PRA de M. Cryptic chromosomal diversity in the complex “Geophagus” brasiliensis (Perciformes, Cichlidae). Zebrafish. 2016;13:33–44.
Pazian MF, Pereira LHG, Shimabukuru-Dias CK, Oliveira C, Foresti F. Cytogenetic and molecular markers reveal the complexity of the genus Piabina Reinhardt, 1867 (Characiformes: Characidae). Neotrop Ichthyol. 2012;10:329–40.
Taylor EB. Species pairs of north temperate freshwater fishes: evolution, taxonomy, and conservation. Rev Fish Biol Fish. 1999;9:299–324.
Volobouev VT, Aniskin VM, Lecompte E, Ducroz J-F. Patterns of karyotype evolution in complexes of sibling species within three genera of African murid rodents inferred from the comparison of cytogenetic and molecular data. Cytogenet Genome Res. 2002;96:261–75.
Oyakawa OT. Family Erythrinidae BT - check list of the freshwater fishes of South and Central America. In: Reis RE, Kullander SO, Ferraris CJ, editors. Porto Alegre: Edipucrs; 2003.
de Oliveira EA, Bertollo LAC, Yano CF, Liehr T, Cioffi M de B. Comparative cytogenetics in the genus Hoplias (Characiformes, Erythrinidae) highlights contrasting karyotype evolution among congeneric species. Mol Cytogenet. 2015;8:56.
Morelli S, Vicari MR, Bertollo LAC. Evolutionary cytogenetics of the Hoplias lacerdae Miranda Ribeiro, 1908 group. A particular pathway concerning the other Erythrinidae fish. Braz J Biol. 2007;67:897–903.
Bertollo LAC. Chromosome evolution in the Neotropical Erythrinidae fish family: an overview BT. In: Pizano E, Ozouf-Costaz C, Foresti F, Kapoor BG, editors. Fish Cytogenet. Enfield: Science Publishers; 2007.
•• Cioffi MDB, Yano CF, Sember A, Bertollo LAC. Chromosomal evolution in lower vertebrates: sex chromosomes in neotropical fishes. Genes. 2017;8:258 This paper provides an update on sex chromosome evolution in fishes.
Bertollo LAC, Born GG, Dergam JA, Fenocchio AS, Moreira-Filho O. A biodiversity approach in the Neotropical fish Hoplias malabaricus. Karyotypic survey, geographic distribution of cytotypes and cytotaxonomic considerations. Chromosom Res. 2000;8:603–13.
de Freitas NL, Al-Rikabi ABH, Bertollo LAC, Ezaz T, Yano CF, de Oliveira EA, et al. Early stages of XY sex chromosomes differentiation in the fish Hoplias malabaricus (Characiformes, Erythrinidae) revealed by DNA repeats accumulation. Curr Genomics. 2018;19:216–26.
de Oliveira EA, Sember A, Bertollo LAC, Yano CF, Ezaz T, Moreira-Filho O, et al. Tracking the evolutionary pathway of sex chromosomes among fishes: characterizing the unique XX/XY1Y2 system in Hoplias malabaricus (Teleostei, Characiformes). Chromosoma. 2018;127:115–28.
Cioffi MB, Martins C, Centofante L, Jacobina U, Bertollo LAC. Chromosomal variability among allopatric populations of Erythrinidae fish Hoplias malabaricus: mapping of three classes of repetitive DNAs. Cytogenet Genome Res. 2009;125:132–41.
Cioffi MB, Liehr T, Trifonov V, Molina WF, Bertollo LAC. Independent sex chromosome evolution in lower vertebrates: a molecular cytogenetic overview in the Erythrinidae fish family. Cytogenet Genome Res. 2013;141:186–94.
Born GG, Bertollo LAC. An XX/XY sex chromosome system in a fish species, Hoplias malabaricus, with a polymorphic NOR-bearing X chromosome. Chromosom Res. 2000;8:111–8.
Santos U, Völcker CM, Belei FA, Cioffi MB, Bertollo LAC, Paiva SR, et al. Molecular and karyotypic phylogeography in the Neotropical Hoplias malabaricus (Erythrinidae) fish in eastern Brazil. J Fish Biol. 2009;75:2326–43.
Sember A, Bertollo LAC, Ráb P, Yano CF, Hatanaka T, de Oliveira EA, et al. Sex chromosome evolution and genomic divergence in the fish Hoplias malabaricus (Characiformes, Erythrinidae). Front Genet. 2018;9:1–12.
Bertollo LAC, Fontes MS, Fenocchio AS, Cano J. The X1X2Y sex chromosome system in the fish Hoplias malabaricus. I. G-, C- and chromosome replication banding. Chromosom Res. 1997;5:493–9.
Bertollo LAC, Mestriner CA. The X1X2Y sex chromosome system in the fish Hoplias malabaricus (Pisces, Erythrinidae). II. Meiotic analyses. Chromosome Res. 1998;6:141–7.
Cioffi MB, Bertollo LAC. Initial steps in XY chromosome differentiation in Hoplias malabaricus and the origin of an X1X2Y sex chromosome system in this fish group. Heredity. 2010;105:554–61.
Lima FCT, Malabarba LR, Buckup PA, da Silva JFP, Vari RP, Harold A, et al. Genera Incertae sedis in Characidae. In: Reis RE, Kullander SO, Ferraris Jr C, editors. Check list Freshw fishes South Cent Am. Porto Alegre: Edipucrs; 2003. p. 106–69.
Fowler HW. Os peixes de água doce do Brasil. Arq Zool. Departmento de Zoologia da Secretaria da Agricultura do Estado de Sao Paulo; 1948;6:1–204.
Moreira-Filho O, Bertollo LAC. Astyanax scabripinnis (Pisces; Characidae): a “species complex”. Brazil J Genet. 1991;14:331–57.
Mizoguchi SMHN, Martins-Santos IC. Cytogenetic and morphometric differences in populations of Astyanax “scabripinnis” (Pisces, Characidae) from Maringá region, PR, Brazil. Genet Mol Biol. 1998;21:55–61.
Azpelicueta MM, Almirón AE, Casciotta JR. Astyanax paris: a new species from the Río Uruguay basin of Argentina (Characiformes, Characidae). Copeia. 2002;2002:1052–6.
Azpelicueta MM, Casciotta JR, Almiron AE. Two new species of the genus Astyanax (Characiformes, Characidae) from the Paraná river basin in Argentina. Rev Suisse Zool. 2002;109:243–59.
Bertaco VA, de Lucena CAS. Two new species of Astyanax (Ostariophysi: Characiformes: Characidae) from eastern Brazil, with a synopsis of the Astyanax scabripinnis species complex. Neotrop Ichthyol. 2006;4:53–60.
Ingenito LFS, Duboc LF. A new species of Astyanax (Ostariophysi: Characiformes: Characidae) from the upper rio Iguaçu basin, southern Brazil. Neotrop Ichthyol. 2014;12:281–90.
Malacrida ACCP, Dias AL, Giuliano-Caetano L. Natural triploidy in Astyanax aff. scabripinnis (Pisces, Characidae) of the Tibagi river bay-PR. Cytologia. 2003;68:267–70.
Moreira-Filho O, Fenocchio AS, Pastori MC, Bertollo LAC. Occurrence of a metacentric macrochromosome B in different species of the genus Astyanax (Pisces, Characidae, Tetragonopterinae). Cytologia. 2001;66:59–64.
Machado SN, Neto MF, Bakkali M, Vicari MR, Artoni RF, de Oliveira C, et al. Natural triploidy and B chromosomes in Astyanax scabripinnis (Characiformes, Characidae): a new occurrence. Caryologia. 2012;65:40–6.
Salvador LB, Moreira-Filho O. B chromosomes in Astyanax scabripinnis (Pisces, Characidae). Heredity. 1992;69:50–6.
Maistro EL, Foresti F, Oliveira C, de Almeida Toledo LF. Occurrence of macro B chromosomes in Astyanax scabripinnis paranae (Pisces, Characiformes, Characidae). Genetica. 1992;87:101–6.
Rocon-Stange EA, Almeida-Toledo LF. Supernumerary B chromosomes restricted to males in Asyanax scabripinnis (Pisces, Characidae). Brazil J Genet. 1993;16:601–15.
Vicente VE, Moreira-Filho O, Camacho JPM. Sex-ratio distortion associated with the presence of a B chromosome in Astyanax scabripinnis (Teleostei, Characidae). Cytogenet Genome Res. 1996;74:70–5.
Néo DM, Moreira-Filho O, Camacho JPM. Altitudinal variation for B chromosome frequency in the characid fish Astyanax scabripinnis. Heredity. 2000;85:136–41.
Sá M d FP d, Fragoso-Moura EN, Fenerich-Verani N, Ferro DA de M. Occurrence of intersexuality in “Lambaris”, Astyanax scabripinnis (Jenyns, 1842), small characids from the Brazilian streams. Brazilian Arch Biol Technol. 2008;51:315–22.
Cornelio D, Castro JP, Santos MH, Vicari MR, de Almeida MC, Moreira-Filho O, et al. Hermaphroditism can compensate for the sex ratio in the Astyanax scabripinnis species complex (Teleostei: Characidae): expanding the B chromosome study model. Rev Fish Biol Fish. 2017;27:681–9.
Holbrook SJ, Schmitt RJ, Messmer V, Brooks AJ, Srinivasan M, Munday PL, et al. Reef fishes in biodiversity hotspots are at greatest risk from loss of coral species. PLoS One. 2015;10:e0124054.
Victor BC. How many coral reef fish species are there? Cryptic diversity and the new molecular taxonomy. In: Mora C, editor. Ecol fishes coral reefs. Cambridge: Cambridge University Press; 2015. p. 76–87.
Eschmeyer WN, Fricke R, Fong JD, Polack DA. Marine fish diversity: history of knowledge and discovery (Pisces). Zootaxa. 2010;2525:19–50.
Molina WF. Chromosomal changes and stasis in marine fish groups. In: Pisano E, Ozouf-Costaz C, Foresti F, Kapoor B, editors. Fish Cytogenet. 1st ed. Enfield: Science Publishers; 2007. p. 69–110.
Benton MJ. Biodiversity on land and in the sea. Geol J. 2001;36:211–30.
Vermeij GJ, Grosberg RK. The great divergence: when did diversity on land exceed that in the sea? Integr Comp Biol. 2010;50:675–82.
Neto CCM, Cioffi MB, Bertollo LAC, Molina WF. Extensive chromosomal homologies and evidence of karyotypic stasis in Atlantic grunts of the genus Haemulon (Perciformes). J Exp Mar Bio Ecol. 2011;401:75–9.
Calado LL, Bertollo LAC, Cioffi MB, Costa GWWF, Jacobina UP, Molina WF. Evolutionary dynamics of rDNA genes on chromosomes of the Eucinostomus fishes: cytotaxonomic and karyoevolutive implications. Genet Mol Res. 2014;13:9951–9.
Brum MJI. Correlações entre a filogenia ea citogenética dos peixes Teleósteos. Rev Bras Genet Monogr. 1995;2:5–42.
Da CGWWF, Cioffi MDB, Bertollo LAC, Molina WF. The evolutionary dynamics of ribosomal genes, histone H3, and transposable Rex elements in the genome of Atlantic snappers. J Hered. 2016;107:173–80.
de Sena DCS, Molina WF. Chromosomal rearrangements associated with pelagic larval duration in Labridae (Perciformes). J Exp Mar Bio Ecol. 2007;353:203–10.
Soares RX, Cioffi MB, Bertollo LAC, Borges AT, Costa GWWF, Molina WF. Chromosomal evolution in large pelagic oceanic apex predators, the barracudas (Sphyraenidae, Percomorpha). Genet Mol Res. 2017;16.
Molina WF, Martinez PA, Bertollo LAC, Bidau CJ. Preferential accumulation of sex and Bs chromosomes in biarmed karyotypes by meiotic drive and rates of chromosomal changes in fishes. An Acad Bras Cienc. 2014;86:1801–12.
Gornung E. Twenty years of physical mapping of major ribosomal RNA genes across the teleosts: a review of research. Cytogenet Genome Res. 2013;141:90–102.
Sochorová J, Garcia S, Gálvez F, Symonová R, Kovařík A. Evolutionary trends in animal ribosomal DNA loci: introduction to a new online database. Chromosoma. 2017;1–10.
Molina WF, Maia-Lima FA, Affonso PRAM. Divergence between karyotypical pattern and speciation events in Serranidae fish (Perciformes). Caryologia. 2002;55:299–305.
Accioly I, Bertollo L, Costa G, Jacobina U, Molina W. Chromosomal population structuring in carangids (Perciformes) between the north-eastern and south-eastern coasts of Brazil. Afr J Mar Sci. 2012;34:383–9.
Amorim KDJ, Cioffi MB, Bertollo LAC, Soares RX, Calado LL, Borges AT, et al. Interregional cytogenetic comparisons in Halichoeres and Thalassoma wrasses (Labridae) of coastal and insular regions of the southwestern Atlantic. Genet Mol Res. 2017;16.
Galetti PM Jr, Molina WF, Affonso PRAM, Aguilar CT. Assessing genetic diversity of Brazilian reef fishes by chromosomal and DNA markers. Genetica. 2006;126:161–77.
Ojima Y, Kashiwagi E. Chromosomal evolution associated with Robertsonian fusion in the genus Dascyllus. Proc Jpn Acad Ser B. 1981;57:368–70.
Molina WF, Galetti-Jr PM. Robertsonian rearrangements in the reef fish Chromis (Perciformes, Pomacentridae) involving chromosomes bearing 5s rRNA genes. Genet Mol Biol. 2002;25:373–7.
Getlekha N, Molina WF, de Bello Cioffi M, Yano CF, Maneechot N, Bertollo LAC, et al. Repetitive DNAs highlight the role of chromosomal fusions in the karyotype evolution of Dascyllus species (Pomacentridae, Perciformes). Genetica. 2016;144:203–11.
Kashiwagi E, Takai A, Ojima Y. Chromosomal distribution of constitutive heterochromatin and nucleolus organizer regions in four Dascyllus fishes (Pomacentridae, Perciformes). Cytologia. 2005;70:345–9.
Vitturi R, Carbone P, Catalano E, Macaluso M. Chromosome polymorphism in Gobius paganellus, Linneo 1758 (Pisces, Gobiidae). Biol Bull. 1984;167:658–68.
Vasil’eva ED, Vasil’ev VP. Morphological variability, chromosome polymorphism, and problems of identifying individual species of gobies of the group Ponticola (Gobiidae) and assessing taxonomic relations of local populations. J Ichthyol. 2016;56:644–55.
Thode G, Giles V, Alvarez MC. Multiple chromosome polymorphism in Gobius paganellus (Teleostei, Perciformes). Heredity. 1985;54:3–7.
de Lima-Filho PA, de Bello Cioffi M, Bertollo LAC, Molina WF. Chromosomal and morphological divergences in Atlantic populations of the frillfin goby Bathygobius soporator (Gobiidae, Perciformes). J Exp Mar Biol Ecol. 2012;434:63–70.
Lima-Filho PA, Rosa R de S, Souza A d S d, da Costa GWWF, de Oliveira C, Molina WF. Evolutionary diversification of Western Atlantic Bathygobius species based on cytogenetic, morphologic and DNA barcode data. Rev Fish Biol Fish. 2016;26:109–21.
Rodríguez-Rey GT, Carvalho Filho A, De Araújo ME, Solé-Cava AM. Evolutionary history of Bathygobius (Perciformes: Gobiidae) in the Atlantic biogeographic provinces: a new endemic species and old mitochondrial lineages. Zool J Linnean Soc. 2018;182:360–84.
Williams SL, Davidson IC, Pasari JR, Ashton GV, Carlton JT, Crafton RE, et al. Managing multiple vectors for marine invasions in an increasingly connected world. Bioscience. 2013;63:952–66.
Maruska KP, Peyton KA. Interspecific spawning between a recent immigrant and an endemic damselfish (Pisces: Pomacentridae) in the Hawaiian Islands. Pac Sci. 2007;61:211–21.
Molina WF, da Costa GWWF, Soares RX, de Mello Affonso PRA, de Bello Cioffi M, de Araújo WC, et al. Extensive chromosome conservatism in Atlantic butterflyfishes, genus Chaetodon Linnaeus, 1758: implications for the high hybridization success. Zool Anz. 2013;253:137–42.
Nirchio M, Ehemann N, Siccha-Ramirez R, Ron E, Pérez JE, Rossi AR, et al. Karyotype of the invasive species Pterois volitans (Scorpaeniformes: Scorpaenidae) from Margarita Island, Venezuela. Rev Biol Trop. 2014;62:1365–73.
Getlekha N, Cioffi M de B, Yano CF, Maneechot N, Bertollo LAC, Supiwong W, et al. Chromosome mapping of repetitive DNAs in sergeant major fishes (Abudefdufinae, Pomacentridae): a general view on the chromosomal conservatism of the genus. Genetica. 2016;144:567–76.
Nalbant TT, Ráb P, Bohlen J, Saitoh K. Evolutionary success of the loaches of the genus Cobitis (Pisces: Ostariophysi: Cobitidae). Trav Mus Natl Hist Nat Grigore Antipa. 2001;43:277–89.
Kottelat M, Freyhof J. Handbook of European freshwater fishes. Publications Kottelat; 2007.
Berg LS. Freshwater fishes of the USSR and neighbouring countries. 1949.
Perdices A, Bohlen J, Šlechtová V, Doadrio I. Molecular evidence for multiple origins of the European spined loaches (Teleostei, Cobitidae). PLoS One. 2016;11:e0144628.
Arai R. Fish karyotypes: a check list. Springer Science & Business Media; 2011.
Janko K, Bohlen J, Lamatsch D, Flajšhans M, Kotlík P, Ráb P, et al. Evidence for gynogenesis as the reproductive mode of hybrid loaches (Cobitis: Teleostei): on the evolution of polyploidy in asexual vertebrates. Genetica. 2007;131:185–94.
Rábová M, Ráb P, Ozouf-Costaz C. Extensive polymorphism and chromosomal characteristics of ribosomal DNA in a loach fish, Cobitis vardarensis (Ostariophysi, Cobitidae) detected by different banding techniques and fluorescence in situ hybridization (FISH). Genetica. 2001;111:413–22.
Boroń A. Banded karyotype of spined loach Cobitis taenia and triploid Cobitis from Poland. Genetica. 1999;105:293–300.
Boroń A. Replication banding patterns in the spined loach, Cobitis taenia L. (Pisces, Cobitidae). Genetica. 2003;119:51–5.
Ráb P, Rábová M, Bohlen J, Lusk S. Genetic differentiation of the two hybrid diploid-polyploid complexes of loaches, genus Cobitis (Cobitidae) involving C. taenia, C. elongatoides and C. spp. in the Czech Republic: karyotypes and cytogenetic diversity. Folia Zool. 2000;49:55–66.
Janko K, Culling MA, Rab P, Kotlik P. Ice age cloning–comparison of the quaternary evolutionary histories of sexual and clonal forms of spiny loaches (Cobitis; Teleostei) using the analysis of mitochondrial DNA variation. Mol Ecol. 2005;14:2991–3004.
Janko K, Kotlik P, Ráb P. Evolutionary history of asexual hybrid loaches (Cobitis: Teleostei) inferred from phylogenetic analysis of mitochondrial DNA variation. J Evol Biol. 2003;16:1280–7.
Vasil’ev VP, Vasil’eva ED. A new diploid-polyploid complex in fishes. Dokl Akad Nauk SSSR. 1982;266:250–2.
Boron A. Karyotype study of diploid and triploid Cobitis taenia (Pisces, Cobitididae) from Vistula river basin. Cytobios. 1992;72:201–6.
Janko K, Bohlen J, Lamatsch D, Flajšhans M, Epplen JT, Ráb P, et al. The gynogenetic reproduction of diploid and triploid hybrid spined loaches (Cobitis: Teleostei), and their ability to establish successful clonal lineages on the evolution of polyploidy in asexual vertebrates. Genetica. 2007;131:185–94.
Choleva L, Apostolou A, Rab P, Janko K. Making it on their own: sperm-dependent hybrid fishes (Cobitis) switch the sexual hosts and expand beyond the ranges of their original sperm donors. Philos Trans R Soc B Biol Sci. 2008;363:2911–9.
Volff JN. Genome evolution and biodiversity in teleost fish. Heredity. 2005;94:280–94.
Froschauer A, Braasch I, Volff J. Fish genomes, comparative genomics and vertebrate evolution. Curr Genomics. 2006;7:43–57.
Amores A, Giles V, Thode G, Alvarez MC. A tandem fusion in the fish Gobius paganellus (Gobiidae, Perciformes), a karyotypically polymorphic species. Genome. 1990;33:57–9.
Symonová R, Flajšhans M, Sember A, Havelka M, Gela D, Kořínková T, et al. Molecular cytogenetics in artificial hybrid and highly polyploid sturgeons: an evolutionary story narrated by repetitive sequences. Cytogenet Genome Res. 2013;141:153–62.
Havelka M, Hulák M, Ráb P, Rábová M, Lieckfeldt D, Ludwig A, et al. Fertility of a spontaneous hexaploid male Siberian sturgeon, Acipenser baerii. BMC Genet. 2014;15:5.
• Havelka M, Bytyutskyy D, Symonová R, Ráb P, Flajšhans M. The second highest chromosome count among vertebrates is observed in cultured sturgeon and is associated with genome plasticity. Genet Sel Evol. 2016;48:12 This paper describes the second highest chromosomal number among vertebrates.
Symonová R, Havelka M, Amemiya CT, Howell WM, Kořínková T, Flajšhans M, et al. Molecular cytogenetic differentiation of paralogs of Hox paralogs in duplicated and re-diploidized genome of the North American paddlefish (Polyodon spathula). BMC Genet. 2017;18:19.
Flajšhans M, Ráb P, Linhart O. Polyploidie a genomové manipulace u ryb. Genetika šlechtění ryb – Kn. VURH JU Vodňany; 2008. p. 153–195.
Crow KD, Smith CD, Cheng J-F, Wagner GP, Amemiya CT. An independent genome duplication inferred from Hox paralogs in the American paddlefish–a representative basal ray-finned fish and important comparative reference. Genome Biol Evol. 2012;4:937–53.
Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364.
Colihueque N, Iturra P, Estay F, Dı́az NF. Diploid chromosome number variations and sex chromosome polymorphism in five cultured strains of rainbow trout (Oncorhynchus mykiss). Aquaculture. 2001;198:63–77.
Schartl M, Schmid M, Nanda I. Dynamics of vertebrate sex chromosome evolution: from equal size to giants and dwarfs. Chromosoma. 2016;125:553–71.
Takehana Y, Demiyah D, Naruse K, Hamaguchi S, Sakaizumi M. Evolution of different Y chromosomes in two medaka species, Oryzias dancena and O. latipes. Genetics. 2007;175:1335–40.
Cioffi MB, Moreira-Filho O, Almeida-Toledo LF, Bertollo LAC. The contrasting role of heterochromatin in the differentiation of sex chromosomes: an overview from Neotropical fishes. J Fish Biol. 2012;80:2125–39.
Uyeno T, Miller RR. Multiple sex chromosomes in a Mexican cyprinodontid fish. Nature. 1971;231:452–3.
Kitano J, Peichel CL. Turnover of sex chromosomes and speciation in fishes. Environ Biol Fish. 2012;94:549–58.
Bracewell RR, Bentz BJ, Sullivan BT, Good JM. Rapid neo-sex chromosome evolution and incipient speciation in a major forest pest. Nat Commun. 2017;8:1593.
Nguyen P, Sýkorová M, Šíchová J, Kůta V, Dalíková M, Čapková Frydrychová R, et al. Neo-sex chromosomes and adaptive potential in tortricid pests. Proc Natl Acad Sci U S A. 2013;110:6931–6.
Kottler VA, Schartl M. The colorful sex chromosomes of teleost fish. Genes. 2018;9:233.
Haaf T, Schmid M. An early stage of ZW/ZZ sex chromosome differentiation in Poecilia sphenops var. melanistica (Poeciliidae, Cyprinodontiformes). Chromosoma. 1984;89:37–41.
Koubová M, Pokorná MJ, Rovatsos M, Farkačová K, Altmanová M, Kratochvíl L. Sex determination in Madagascar geckos of the genus Paroedura (Squamata: Gekkonidae): are differentiated sex chromosomes indeed so evolutionary stable? Chromosom Res. 2014;22:441–52.
Liehr T. Classical cytogenetics “is not equal to” banding cytogenetics. Mol Cytogenet. 2017 Feb 16;10:3.
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq (Proc. Nos. 401962/2016-4 and 304992/2015-1), Fundação de Amparo à Pesquisa do Estado de São Paulo–FAPESP (Proc. Nos. 2016/21411-7 and 2016/22196-2), and CAPES/Alexander von Humboldt (Proc. No. 88881.136128/2017-01). PR and AS were supported by the project EXCELLENCE CZ.02.1.01/0.0/0.0/15_003/0000460 OP RDE and RVO: 67985904.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors reported no potential conflicts of interest relevant to this article.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards.
Additional information
This article is part of the Topical Collection on Cytogenetics
Rights and permissions
About this article
Cite this article
de Bello Cioffi, M., Moreira-Filho, O., Ráb, P. et al. Conventional Cytogenetic Approaches—Useful and Indispensable Tools in Discovering Fish Biodiversity. Curr Genet Med Rep 6, 176–186 (2018). https://doi.org/10.1007/s40142-018-0148-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40142-018-0148-7