Abstract
Purpose of Review
This review outlines a perioperative approach to the pediatric patient with a seizure disorder and anesthesia for minimally invasive epilepsy surgical treatment.
Recent Findings
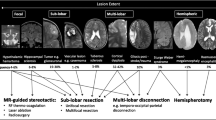
Surgical interventions for epilepsy have undergone dramatic progression. Minimally invasive treatments and high-tech imaging, such as intraoperative magnetic resonance imaging (iMRI), are recent advances. Many anesthetics have epileptogenic activity and must be adjusted for an effective anesthetic. The needs for imaging techniques, moving patients in and out of computer tomography (CT) and magnetic resonance imaging (MRI) locations, require careful planning and management.
Summary
Antiepileptic medications interact with anesthetic agents, and common anesthetics can precipitate or suppress seizure activity. Furthermore, neurosurgical interventions continue to evolve with iMRI, robotic, and minimally invasive techniques. The pediatric anesthesiologist will need to adapt and adjust anesthetic choices to enhance seizure foci localization and treatment.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Engel J. The current place of epilepsy surgery. Curr Opin Neurol. 2018;31(2):192–7.
Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol. 2012;8(12):669–77.
Jayalakshmi S, Vooturi S, Gupta S, Panigrahi M. Epilepsy surgery in children. Neurol India. 2017;65(3):9.
Soriano SG, Bozza P. Anesthesia for epilepsy surgery in children. Childs Nerv Syst. 2006;22(8):834–43.
Valencia I, Pfeifer H, Thiele EA. General anesthesia and the ketogenic diet: clinical experience in nine patients. Epilepsia. 2002;43(5):525–9.
•• Conover ZR, Talai A, Klockau KS, Ing RJ, Chatterjee D. Perioperative management of children on ketogenic dietary therapies. Anesth Analg. 2020;131(6):1872–82 Detailed review and summary of ketogenic diets, including medication lists with carbohydrate content.
Simpson AK, Levy N, Hall GM. Peri-operative iv fluids in diabetic patients – don’t forget the salt. Anaesthesia. 2008;63(10):1043–5.
Billiodeaux ST, Samuelson CG, Willett O, Arulkumar S, Thomas D, Hamilton CS, et al. Intraoperative and postoperative blood glucose concentrations in diabetic surgical patients receiving lactated Ringer’s versus normal saline: a retrospective review of medical records. Ochsner J. 2014;14(2):175–8.
Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear T, Vender JS, et al. The effect of single low-dose dexamethasone on blood glucose concentrations in the perioperative period: a randomized, placebo-controlled investigation in gynecologic surgical patients. Anesth Analg. 2014;118(6):1204–12.
Soysal E, Gries H, Wray C. Pediatric patients on ketogenic diet undergoing general anesthesia-a medical record review. J Clin Anesth. 2016;35:170–5.
Ouchi K, Sugiyama K. Required propofol dose for anesthesia and time to emerge are affected by the use of antiepileptics: prospective cohort study. BMC Anesthesiol. 2015;15(1):1–7.
Eker HE, Cok OY, Aribogan A, Arslan G. Children on phenobarbital monotherapy requires more sedatives during MRI. Pediatr Anesth. 2011;21(10):998–1002.
Zhou S-F, Zhou Z-W, Yang L-P, Cai J-P. Substrates, inducers, inhibitors and structure-activity relationships of human cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480–675.
Ouchi K. The number and kind of antiepileptics affect propofol dose requirement for anesthesia: observational study. Odontology. 2020;108(1):102–8.
Sumpter A, Anderson BJ. Phenobarbital and some anesthesia implications. Pediatr Anesth. 2011;21(10):995–7.
Maeda S, Tomoyasu Y, Higuchi H, Ishii-Maruhama M, Egusa M, Miyawaki T. Independent predictors of delay in emergence from general anesthesia. Anesth Prog. 2015;62(1):8–13.
Ghodke-Puranik Y, Thorn CF, Lamba JK, Leeder JS, Song W, Birnbaum AK, et al. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2013;23(4):236–41.
Dhillon S, Richens A. Valproic acid and diazepam interaction in vivo. Br J Clin Pharmacol. 1982;13(4):553–60.
Abdallah C. Considerations in perioperative assessment of valproic acid coagulopathy. J Anaesthesiol-Clin Pharmacol. 30(1):7–9.
Gerstner T, Teich M, Bell N, Longin E, Dempfle C-E, Brand J, et al. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia. 2006;47(7):1136–43.
Chambers HG, Weinstein CH, Mubarak SJ, Wenger DR, Silva PD. The effect of valproic acid on blood loss in patients with cerebral palsy. J Pediatr Orthop. 1999;19(6):792–5.
Ward MM, Barbaro NM, Laxer KD, Rampil IJ. Preoperative valproate administration does not increase blood loss during temporal lobectomy. Epilepsia. 1996;37(1):98–101.
Anderson GD, Lin Y-X, Berge C, Ojemann GA. Absence of bleeding complications in patients undergoing cortical surgery while receiving valproate treatment. J Neurosurg. 1997;87(2):252–6.
Kreuz W, Mentzer D, Becker S, Scharrer I, Kornhuber B. Haemate P® in children with von Willebrand’s disease. Pathophysiol Haemost Thromb. 1994;24(5):304–10.
Gidal B, Spencer N, Maly M, Pitterle M, Williams E, Collins M, et al. Valproate-mediated disturbances of hemostasis: relationship to dose and plasma concentration. Neurology. 1994;44(8):1418–8.
Bajwa SJS, Jindal R. Epilepsy and nonepilepsy surgery: recent advancements in anesthesia management. Anesth Essays Res. 2013;7(1):10–7.
Soriano SG, Kaus SJ, Sullivan LJ, Martyn JA. Onset and duration of action of rocuronium in children receiving chronic anticonvulsant therapy. Paediatr Anaesth. 2000;10(2):133–6.
Soriano SG, Sullivan LJ, Venkatakrishnan K, Greenblatt DJ, Martyn JAJ. Pharmacokinetics and pharmacodynamics of vecuronium in children receiving phenytoin or carbamazepine for chronic anticonvulsant therapy. BJA Br J Anaesth. 2001;86(2):223–9.
Wright PMC, McCarthy G, Szenohradszky J, Sharma ML, Caldwell JE. Influence of chronic phenytoin administration on the pharmacokinetics and pharmacodynamics of Vecuronium. Anesthesiology. 2004;100(3):626–33.
Guldiken B, Rémi J, Noachtar S. Cardiovascular adverse effects of phenytoin. J Neurol. 2016;263(5):861–70.
Perks A, Cheema S, Mohanraj R. Anaesthesia and epilepsy. Br J Anaesth. 2012;108(4):562–71.
Faucette SR, Wang H, Hamilton GA, Jolley SL, Gilbert D, Lindley C, et al. Regulation of Cyp2b6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32(3):348–58.
Patsalos PN, Fröscher W, Pisani F, Van Rijn CM. The importance of drug interactions in epilepsy therapy. Epilepsia. 2002;43(4):365–85.
Hayashi T, Higuchi H, Tomoyasu Y, Ishii-Maruhama M, Maeda S, Miyawaki T. Effect of carbamazepine or phenytoin therapy on blood level of intravenously administered midazolam: a prospective cohort study. J Anesth. 2016;30(1):166–9.
Sobotka JL, Alexander B, Cook BL. A review of carbamazepine’s hematologic reactions and monitoring recommendations. DICP. 1990;24(12):1214–9.
Buck ML, Goodkin HP. Use of lacosamide in children with refractory epilepsy. J Pediatr Pharmacol Ther JPPT. 2012;17(3):211–9.
Welsh SS, Lin N, Topjian AA, Abend NS. Safety of intravenous lacosamide in critically ill children. Seizure. 2017;52:76–80.
Ortiz de la Rosa JS, Ladino LD, Rodríguez PJ, Rueda MC, Polanía JP, Castañeda AC. Efficacy of lacosamide in children and adolescents with drug-resistant epilepsy and refractory status epilepticus: a systematic review. Seizure. 2018;56:34–40.
Farkas V, Steinborn B, Flamini JR, Zhang Y, Yuen N, Borghs S, et al. Efficacy and tolerability of adjunctive lacosamide in pediatric patients with focal seizures. Neurology. 2019;93(12):e1212–26.
Hanrahan B, Carson RP. Felbamate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2020 Nov 15]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK539799/
Thakkar K, Billa G, Rane J, Chudasama H, Goswami S, Shah R. The rise and fall of felbamate as a treatment for partial epilepsy--aplastic anemia and hepatic failure to blame? Expert Rev Neurother. 2015;15(12):1373–5.
Shah YD, Singh K, Friedman D, Devinsky O, Kothare SV. Evaluating the safety and efficacy of felbamate in the context of a black box warning: a single center experience. Epilepsy Behav. 2016;56:50–3.
Faught E. Topiramate in the treatment of partial and generalized epilepsy. Neuropsychiatr Dis Treat. 2007;3(6):811–21.
Takeoka M, Riviello JJ, Pfeifer H, Thiele EA. Concomitant treatment with topiramate and ketogenic diet in pediatric epilepsy. Epilepsia. 2002;43(9):1072–5.
Barnett SM, Jackson AH, Rosen BA, Garb JL, Braden GL. Nephrolithiasis and nephrocalcinosis from topiramate therapy in children with epilepsy. Kidney Int Rep. 2018;3(3):684–90.
Mufson JM. Lamotrigine: pharmacology, clinical utility, and new safety concerns. Am J Psychiatry Resid J. 2018;13(12):2–4.
Bonicalzi V, Canavero S, Cerutti F, Piazza M, Clemente M, Chió A. Lamotrigine reduces total postoperative analgesic requirement: a randomized double-blind, placebo-controlled pilot study. Surgery. 1997;122(3):567–70.
Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, et al. Opioid receptor antagonism attenuates antidepressant effects of ketamine. Am J Psychiatry. 2018;175(12):1205–15.
Kornhall D, Nielsen EW. Failure of ketamine anesthesia in a patient with lamotrigine overdose. Case Rep Crit Care [Internet]. 2014 [cited 2020 Nov 15];2014. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4119912/
Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270–6.
Kwan S-Y, Chuang Y-C, Huang C-W, Chen T-C, Jou S-B, Dash A. Zonisamide: review of recent clinical evidence for treatment of epilepsy. CNS Neurosci Ther. 2015;21(9):683–91.
Deshpande LS, DeLorenzo RJ. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front Neurol [Internet]. 2014 Jan 31 [cited 2020 Nov 15];5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3907711/
Archer DP, Lamberty Y, Wang B, Davis MJ, Samanani N, Roth SH. Levetiracetam reduces anesthetic-induced hyperalgesia in rats. Anesth Analg. 2007;104(1):180–5.
Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Pediatr Anesth. 2005;15(4):266–74.
Constant I, Dubois M-C, Piat V, Moutard M-L, McCue M, Murat I. Changes in electroencephalogram and autonomic cardiovascular activity during induction of anesthesia with sevoflurane compared with halothane in children. Anesthesiology. 1999;91(6):1604–4.
Gibert S, Sabourdin N, Louvet N, Moutard M-L, Piat V, Guye M-L, et al. Epileptogenic effect of sevoflurane: determination of the minimal alveolar concentration of sevoflurane associated with major epileptoid signs in children. Anesthesiology. 2012;117(6):1253–61.
Hisada K, Morioka T, Fukui K, Nishio S, Kuruma T, Irita K, et al. Effects of sevoflurane and isoflurane on electrocorticographic activities in patients with temporal lobe epilepsy. J Neurosurg Anesthesiol. 2001;13(4):333–7.
Butterworth JF, Mackey DC, Wasnick JD. Chapter 28. Anesthesia for patients with neurologic & psychiatric diseases. In: Morgan & Mikhail’s Clinical Anesthesiology [Internet]. 5th ed. New York, NY: The McGraw-Hill Companies; 2013 [cited 2020 Nov 29]. Available from: accessmedicine.mhmedical.com/content.aspx?aid=57235035.
Tempelhoff R, Modica PA, Spitznagel EL. Anticonvulsant therapy increases fentanyl requirements during anaesthesia for craniotomy. Can J Anaesth. 1990;37(3):327–32.
Hamano S, Sugiyama N, Yamashita S, Tanaka M, Hayakawa M, Minamitani M, et al. Intravenous lidocaine for status epilepticus during childhood. Dev Med Child Neurol. 2006;48(3):220–2.
Yildiz B, Citak A, Uçsel R, Karaböcüoğlu M, Aydinli N, Uzel N. Lidocaine treatment in pediatric convulsive status epilepticus. Pediatr Int Off J Jpn Pediatr Soc. 2008;50(1):35–9.
• Hubert A. Benzon. Epilepsy surgery. In: Essentials of Pediatric Neuroanesthesia. Cambridge University PRess; 2018. p. 102–11. Details the anesthetic implications for patients with epilepsy undergoing surgical treatment for epilepsy foci.
Modica PA, Tempelhoff R, White PF. Pro- and anticonvulsant effects of anesthetics (part II). Anesth Analg. 1990;70(4):433–44.
Skoch J, Adelson PD, Bhatia S, Greiner HM, Rydenhag B, Scavarda D, et al. Subdural grid and depth electrode monitoring in pediatric patients. Epilepsia. 2017;58(S1):56–65.
Soriano SG, Eldredge EA, Wang FK, Kull L, Madsen JR, Black PM, et al. The effect of propofol on intraoperative electrocorticography and cortical stimulation during awake craniotomies in children. Pediatr Anesth. 2000;10(1):29–34.
Rickerson EM, Crossley LJ. Craniotomy. In: Vacanti CA, Sikka P, Urman R, Segal BS, editors. Essential Clinical Anesthesia [Internet]. Cambridge: Cambridge University Press; 2011 [cited 2020 Dec 19]. p. 591–6. Available from: https://www.cambridge.org/core/product/identifier/9780511842306%23c72020-6007/type/book_part
Tanaka S, Oda Y, Ryokai M, Uda T, Kunihiro N, Kuki I, et al. The effect of sevoflurane on electrocorticographic spike activity in pediatric patients with epilepsy. Pediatr Anesth. 2017;27(4):409–16.
Lewis EC, Weil AG, Duchowny M, Bhatia S, Ragheb J, Miller I. MR-guided laser interstitial thermal therapy for pediatric drug-resistant lesional epilepsy. Epilepsia. 2015;56(10):1590–8.
Perry MS, Donahue DJ, Malik SI, Keator CG, Hernandez A, Reddy RK, et al. Magnetic resonance imaging–guided laser interstitial thermal therapy as treatment for intractable insular epilepsy in children. J Neurosurg Pediatr. 2017;20(6):575–82.
Fayed I, Sacino MF, Gaillard WD, Keating RF, Oluigbo CO. MR-guided laser interstitial thermal therapy for medically refractory lesional epilepsy in pediatric patients: experience and outcomes. Pediatr Neurosurg. 2018;53(5):322–9.
• Arocho-Quinones EV, Lew SM, Handler MH, Tovar-Spinoza Z, Smyth M, Bollo R, et al. Magnetic resonance–guided stereotactic laser ablation therapy for the treatment of pediatric brain tumors: a multiinstitutional retrospective study. J Neurosurg Pediatr. 2020;26(1):13–21 Multi Institutional study of 89 patients in the surgical literature who had minimally invasive laser ablation therapy for epilepsy.
Xia Z, Wu X, Li J, Liu Z, Chen F, Zhang L, et al. Minimally invasive surgery is superior to conventional craniotomy in patients with spontaneous Supratentorial intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2018;115:266–73.
•• Levin DN, McClain CD, Stone SSD, Madsen JR, Soriano S. Anesthetic management and outcomes for MRI-guided laser interstitial thermal therapy (LITT) for seizure focus in pediatrics: a single centre experience with 10 consecutive patients. Pediatr Anesth [Internet]. [cited 2020 Oct 18];n/a(n/a). Available from: http://onlinelibrary.wiley.com/doi/abs/10.1111/pan.13929First case series at a single institution of pediatric patients undergoing minimally invasive surgery for epilepsy
Acknowledgements
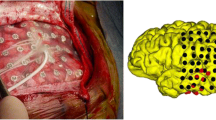
The authors would like to thank neurosurgeon, Dr. Jonathon James Parker, M.D. Ph.D., for his surgical image and expert consultation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Anesthesia
Rights and permissions
About this article
Cite this article
Wong, B.J., Agarwal, R. & Chen, M.I. Anesthesia for the Pediatric Patient With Epilepsy and Minimally Invasive Surgery for Epilepsy. Curr Anesthesiol Rep 11, 233–242 (2021). https://doi.org/10.1007/s40140-021-00457-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-021-00457-2