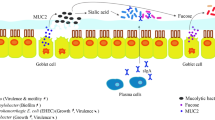
Abstract
Purpose of Review
Our perception of human microbes has changed greatly in the past decade from a focus on pathogens and infections to a new world view of mutualism and coevolution of microbes and mammalian hosts. This review article seeks to explain the dynamic interactions occurring between the intestinal microbiome and the mammalian host mucosa..
Recent Findings
Microbial metabolites influence the functions of epithelial, endothelial, and immune cells in the intestinal mucosa. Microbial metabolites like SCFAs and B complex vitamins influence macrophage differentiation and polarization, whereas microbe-derived biogenic amines such as histamine modulate the biology of the intestinal epithelium and immune responses. Aberrant bacterial lipopolysaccharide-mediated signaling may be involved in the pathogenesis of chronic intestinal inflammation and colorectal carcinogenesis.
Summary
We conclude that gut microbes (commensals and probiotics) can have profound impact on mammals by modulation of intestinal immunity and physiology and by influencing the functions of various cell types within the intestine. In addition, microbial metabolites have well-defined effects on shaping the gut epithelium, and these compounds play a key role in maintaining intestinal homeostasis. Therefore, effectively manipulating the microbiome via changes in diet and microbial composition and function may yield advances regarding diagnosis and treatment.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tropini C, Earle KA, Huang KC, Sonnenburg JL. The gut microbiome: connecting spatial organization to function. Cell Host Microbe. 2017;21(4):433–42. https://doi.org/10.1016/j.chom.2017.03.010.
van den Elsen LW, Poyntz HC, Weyrich LS, Young W, Forbes-Blom EE. Embracing the gut microbiota: the new frontier for inflammatory and infectious diseases. Clin Transl Immunol. 2017;6(1):e125. https://doi.org/10.1038/cti.2016.91.
Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr Res. 2014;76:2–10.
Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18(1):2. https://doi.org/10.1186/s12865-016-0187-3.
Mariat D, Firmesse O, Levenez F, Guimaraes V, Sokol H, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123.
Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, et al. The phylogeny of the genus clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44(4):812–26. https://doi.org/10.1099/00207713-44-4-812.
Rajilic-Stojanovic M, Smidt H, de Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9:2125–36.
Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, et al. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68(7):3401–7. https://doi.org/10.1128/AEM.68.7.3401-3407.2002.
Booijink CC, Zoetendal EG, Kleerebezem M, de Vos WM. Microbial communities in the human small intestine: coupling diversity to metagenomics. Future Microbiol. 2007;2(3):285–95. https://doi.org/10.2217/17460913.2.3.285.
Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4659–65. https://doi.org/10.1073/pnas.1006451107.
Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–9.
Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12(5):319–30. https://doi.org/10.1007/s11894-010-0131-2.
Ermund A, Gustafsson JK, Hansson GC, Keita AV. Mucus properties and goblet cell quantification in mouse, rat and human ileal Peyer’s patches. PLoS One. 2013;8(12):e83688. https://doi.org/10.1371/journal.pone.0083688.
Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G922–9. https://doi.org/10.1152/ajpgi.2001.280.5.G922.
• Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. This study clearly demonstrated on how two different commensals can interact with each other and how that interaction can benefit host health. This study clearly stands out as an example that complex bacterial interactions are important.
Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1408–25. https://doi.org/10.1016/j.bbamem.2006.03.030.
Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, et al. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281(4):2005–11. https://doi.org/10.1074/jbc.M511044200.
Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J Clin Invest. 2002;109(6):693–7. https://doi.org/10.1172/JCI0215218.
Schmidtchen A, Frick IM, Andersson E, Tapper H, Bjorck L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol. 2002;46(1):157–68. https://doi.org/10.1046/j.1365-2958.2002.03146.x.
Yan F, Cao H, Cover TL, Whitehead R, Washington MK, et al. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–75.
Gao C, Major A, Rendon D, Lugo M, Jackson V, et al. Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. MBio. 2015;6:e01358–15.
Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7:e31951.
•• Ganesh BP, Hall A, Ayyaswamy S, Nelson JW, Fultz R, et al. (2017) Diacylglycerol kinase synthesized by commensal Lactobacillus reuteri diminishes protein kinase C phosphorylation and histamine-mediated signaling in the mammalian intestinal epithelium. Mucosal Immunol. This study provides a thorough description of microbial metabolites and microbial proteins communicating with the intestinal epithelium and balancing immune homeostasis via molecular signaling.
• Gao C, Ganesh BP, Shi Z, Shah RR, Fultz R, Major A, et al. Gut microbe-mediated suppression of inflammation-associated colon carcinogenesis by luminal histamine production. Am J Pathol. 2017;187(10):2323–36. https://doi.org/10.1016/j.ajpath.2017.06.011. This study showed that microbial metabolites can influence and reduce the colon tumor burden.
Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. https://doi.org/10.1186/s12967-017-1175-y.
Bailey MT. The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation. Horm Behav. 2012;62(3):286–94. https://doi.org/10.1016/j.yhbeh.2012.02.006.
Sekirov I, Finlay BB. The role of the intestinal microbiota in enteric infection. J Physiol. 2009;587(17):4159–67. https://doi.org/10.1113/jphysiol.2009.172742.
Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends Microbiol. 2008;16(3):107–14. https://doi.org/10.1016/j.tim.2007.12.008.
Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, et al. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5(10):2177–89. https://doi.org/10.1371/journal.pbio.0050244.
Santos RL. Pathobiology of Salmonella, intestinal microbiota, and the host innate immune response. Front Immunol. 2014;5(252). https://doi.org/10.3389/fimmu.2014.00252.
Thiennimitr P, Winter SE, Baumler AJ. Salmonella, the host and its microbiota. Curr Opin Microbiol. 2012;15(1):108–14. https://doi.org/10.1016/j.mib.2011.10.002.
Kurth JM, Dahl C, Butt JN. Catalytic protein film electrochemistry provides a direct measure of the tetrathionate/thiosulfate reduction potential. J Am Chem Soc. 2015;137:13232–5.
Liu YW, Denkmann K, Kosciow K, Dahl C, Kelly DJ. Tetrathionate stimulated growth of Campylobacter jejuni identifies a new type of bi-functional tetrathionate reductase (TsdA) that is widely distributed in bacteria. Mol Microbiol. 2013;88:173–88.
Swart AL, Hensel M. Interactions of Salmonella enterica with dendritic cells. Virulence. 2012;3(7):660–7. https://doi.org/10.4161/viru.22761.
Deng W, Marshall NC, Rowland JL, McCoy JM, Worrall LJ, Santos AS, et al. Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol. 2017;15(6):323–37. https://doi.org/10.1038/nrmicro.2017.20.
Loetscher Y, Wieser A, Lengefeld J, Kaiser P, Schubert S, et al. Salmonella transiently reside in luminal neutrophils in the inflamed gut. PLoS One. 2012;7:e34812.
Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc Natl Acad Sci U S A. 2012;109:1269–74.
Ganesh BP, Klopfleisch R, Loh G, Blaut M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS One. 2013;8(9):e74963. https://doi.org/10.1371/journal.pone.0074963.
Joeris T, Muller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 2017;10(4):845–64. https://doi.org/10.1038/mi.2017.22.
Labonte AC, Tosello-Trampont AC, Hahn YS. The role of macrophage polarization in infectious and inflammatory diseases. Mol Cells. 2014;37(4):275–85. https://doi.org/10.14348/molcells.2014.2374.
•• Ji J, Shu D, Zheng M, Wang J, Luo C, et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci Rep. 2016;6:24838. This study explained that microbial metabolites strongly influence the macrophage polarization.
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52. https://doi.org/10.1038/nri.2016.42.
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–39.
LeBlanc JG, Chain F, Martin R, Bermudez-Humaran LG, Courau S, et al. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Factories. 2017;16(79):79. https://doi.org/10.1186/s12934-017-0691-z.
Mazur-Bialy AI, Buchala B, Plytycz B. Riboflavin deprivation inhibits macrophage viability and activity—a study on the RAW 264.7 cell line. Br J Nutr. 2013;110:509–14.
Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113(2):438–46. https://doi.org/10.1182/blood-2008-04-150789.
Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. https://doi.org/10.1038/nri2653.
Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41(10):751–90. https://doi.org/10.2165/00003088-200241100-00005.
Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, et al. A gut–vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–4.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. https://doi.org/10.1016/j.nbd.2009.07.030.
Sun J, Hobert ME, Rao AS, Neish AS, Madara JL. Bacterial activation of beta-catenin signaling in human epithelia. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G220–7. https://doi.org/10.1152/ajpgi.00498.2003.
Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443(7111):548–52. https://doi.org/10.1038/nature05124.
Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells—conditional innate immune cells. J Hematol Oncol. 2013;6:61.
Kim JH, Yoon YJ, Lee J, Choi EJ, Yi N, Park KS, et al. Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo. PLoS One. 2013;8(3):e59276. https://doi.org/10.1371/journal.pone.0059276.
Soult MC, Lonergan NE, Shah B, Kim WK, Britt LD, Sullivan CJ. Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells. J Surg Res. 2013;184(1):458–66. https://doi.org/10.1016/j.jss.2013.05.035.
Maurer K, Reyes-Robles T, Alonzo F 3rd, Durbin J, Torres VJ, et al. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe. 2015;17(4):429–40. https://doi.org/10.1016/j.chom.2015.03.001.
Lubkin A, Torres VJ. Bacteria and endothelial cells: a toxic relationship. Curr Opin Microbiol. 2017;35:58–63. https://doi.org/10.1016/j.mib.2016.11.008.
Vikram A, Kim YR, Kumar S, Li Q, Kassan M, Jacobs JS, et al. Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1. Nat Commun. 2016;7:12565. https://doi.org/10.1038/ncomms12565.
Mortensen EM, Nakashima B, Cornell J, Copeland LA, Pugh MJ, Anzueto A, et al. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis. 2012;55(11):1466–73. https://doi.org/10.1093/cid/cis733.
Vandermeer ML, Thomas AR, Kamimoto L, Reingold A, Gershman K, Meek J, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205(1):13–9. https://doi.org/10.1093/infdis/jir695.
Patel JM, Snaith C, Thickett DR, Linhartova L, Melody T, Hawkey P, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS trial). Crit Care. 2012;16(6):R231. https://doi.org/10.1186/cc11895.
Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99(24):15451–5. https://doi.org/10.1073/pnas.202604299.
Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4(3):269–73. https://doi.org/10.1038/ni888.
Chidlow JH Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G5–G18. https://doi.org/10.1152/ajpgi.00107.2007.
Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25(4):201–9. https://doi.org/10.1016/j.it.2004.02.006.
Schirbel A, Kessler S, Rieder F, West G, Rebert N, et al. Pro-angiogenic activity of TLRs and NLRs: a novel link between gut microbiota and intestinal angiogenesis. Gastroenterology. 2013;144(613–623):e619.
•• Alkim C, Alkim H, Koksal AR, Boga S, Sen I. Angiogenesis in inflammatory bowel disease. Int J Inflamm. 2015;2015:970890. This interesting observation shows that activation of bacterial ligands on endothelium resulted in angiogenesis. Therefore, anti-angiogenetic therapy could be used for treatment of IBD.
Acknowledgements
This work was supported by the National Institutes of Health to J.V., including the Texas Medical Center Digestive Disease Center (P30 DK56338), National Cancer Institute (U01 CA170930), and unrestricted research support from BioGaia AB (Stockholm, Sweden) (J.V.). We thank Karen Prince for assisting with graphics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
B.P.G. has a patent P1344SE00 pending to J.V. and B.P.G. as Inventors. Filed by BioGaia AB.
J.V. reports grants for unrestricted research support from BioGaia during the conduct of the study and shares as a member of the advisory board from Diversigen, outside the submitted work.
R.F. and S.A. declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Microbiome and Tissue Homeostasis
Rights and permissions
About this article
Cite this article
Ganesh, B.P., Fultz, R., Ayyaswamy, S. et al. Microbial Interactions with the Intestinal Epithelium and Beyond: Focusing on Immune Cell Maturation and Homeostasis. Curr Pathobiol Rep 6, 47–54 (2018). https://doi.org/10.1007/s40139-018-0165-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-018-0165-y