Abstract
Purpose of Review
The purpose of this review is to provide an updated perspective on directing human pluripotent stem cells (hPSCs) to hepatocyte-like-cells (HLCs) and the associated challenges.
Recent Findings
Recent advances in the hepatocyte differentiation field have largely been focused on increasing the reproducibility and definition of culture systems to further their translation to a clinical setting. There have been advances using new extracellular matrices such as human laminins, and recent work using small molecules to drive the differentiation process has dramatically reduced the cost of producing HLCs with equivalent phenotypes to growth factor-derived cells.
Summary
There are still several key aspects that remain unresolved, including the immature phenotype of hPSC-derived HLCs (a major hurdle for hPSC-derived progeny). Another key question is the zonal identity of the HLCs produced in vitro, which will have major implications in terms of disease modeling and drug metabolism. To date, there has been little investigation of this aspect of hepatic biology reported in the field.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Taylor J, Wilmut I, Sullivan G. What are the limits to cell plasticity? Cell Res. 2010;20:502–3. doi:10.1038/cr.2010.59.
Schwartz SD, Hubschman J-P, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–20. doi:10.1016/S0140-6736(12)60028-2.
Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2014;385:509–16. doi:10.1016/S0140-6736(14)61376-3.
Turner M, Leslie S, Martin NG, et al. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell. 2013;13:382–4. doi:10.1016/j.stem.2013.08.003.
Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17:194–200. doi:10.1038/nrm.2016.10.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi:10.1038/292154a0.
Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8.
Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi:10.1126/science.282.5391.1145.
Knoppers B, Isasi R. Mind the gap: policy approaches to embryonic stem cell and cloning research in 50 countries. Eur J Health Law. 2006;13:9–25. doi:10.1163/157180906777036328.
Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848–57. doi:10.1073/pnas.1200250109.
Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–75. doi:10.1038/nprot.2012.150.
Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–60. doi:10.1038/nmeth.2999.
Toivonen S, Ojala M, Hyysalo A, et al. Comparative analysis of targeted differentiation of human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells reveals variability associated with incomplete transgene silencing in retrovirally derived hiPSC lines. Stem Cells Transl Med. 2013;2:83–93. doi:10.5966/sctm.2012-0047.
Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80. doi:10.1038/nbt.1529.
Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–39. doi:10.1016/j.cell.2014.09.040.
Hay DC, Fletcher J, Payne C, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci U S A. 2008;105:12301–6. doi:10.1073/pnas.0806522105.
• Sullivan GJ, Hay DC, Park I-H, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–35. doi:10.1002/hep.23335. This work from Sullivan and colleagues represents the first instance of hepatocyte-like cells derived from human-induced pluripotent stem cells, and importantly from ethnically diverse backgrounds, opening the possibilities to study drug metabolism in a wide spectrum of the human population.
Hay DC, Zhao D, Fletcher J, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi:10.1634/stemcells.2007-0718.
• Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi:10.1002/hep.23354. This paper demonstrated for the first time that human-induced pluripotent stem cells could be efficiently differentiated to hepatocyte-like-cells.
Song Z, Cai J, Liu Y, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–42. doi:10.1038/cr.2009.107.
Touboul T, Hannan NRF, Corbineau S, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–65. doi:10.1002/hep.23506.
Hannan NRF, Segeritz C-P, Touboul T, Vallier L. Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc. 2013;8:430–7.
Siller R, Greenhough S, Naumovska E, Sullivan GJ. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Rep. 2015;4:939–52. doi:10.1016/j.stemcr.2015.04.001.
• Siller R, Naumovska E, Mathapati S, et al. Development of a rapid screen for the endodermal differentiation potential of human pluripotent stem cell lines. Sci Rep. 2016;6:37178. doi:10.1038/srep37178. This work demonstrated the first report of a small molecule-driven, growth factor-free approach to generate hPSC-derived hepatocyte-like cells, representing a significant (10-fold) reduction in the cost compared to growth factor-based approaches.
Mathapati S, Siller R, Impellizzeri AAR, et al (2016) Small-molecule-directed hepatocyte-like cell differentiation of human pluripotent stem cells. In: Curr. Protoc. Stem Cell Biol. Wiley., Hoboken, p 1G.6.1-1G.6.18.
de Wert G, Mummery C. Human embryonic stem cells: research, ethics and policy. Hum Reprod. 2003;18:672–82. doi:10.1093/HUMREP/DEG143.
Brivanlou AH, Gage FH, Jaenisch R, et al. Setting standards for human embryonic stem cells. Science. 2003;300:913–6. doi:10.1126/science.1082940.
Robertson JA. Human embryonic stem cell research: ethical and legal issues. Nat Rev Genet. 2001;2:74–8. doi:10.1038/35047594.
Daley GQ, Richter LA, Auerbach JM, et al. ETHICS: the ISSCR guidelines for human embryonic stem cell research. Science. 2007;315:603–4. doi:10.1126/science.1139337.
Kastenberg ZJ, Odorico JS. Alternative sources of pluripotency: science, ethics, and stem cells. Transplant Rev. 2008;22:215–22. doi:10.1016/j.trre.2008.04.002.
Briggs R, King TJ. Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc Natl Acad Sci U S A. 1952;38:455–63.
Elsdale TR, Gurdon JB, Fischberg M. A description of the technique for nuclear transplantation in Xenopus laevis. J Embryol Exp Morpholog. 1960;8:437–44.
Gurdon JB. Factors responsible for the abnormal development of embryos obtained by nuclear transplantation in Xenopus laevis. J Embryol Exp Morpholog. 1960;8:327–40.
• Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. This is the first report on the use of expression of a master regulator gene to drive cellular reprogramming and fate change, which laid the ground work for the subsequent work of Yamanaka.
Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–3. doi:10.1038/385810a0.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi:10.1016/j.cell.2006.07.024.
• Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi:10.1016/j.cell.2007.11.019. This work by Takahashi and colleagues demonstrated for the first time that the protocol for generating induced pluripotent stem cells could be applied to human somatic cells.
• Park I-H, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86. doi:10.1016/j.cell.2008.07.041. In this work by Park and colleagues, they showed the first instances of induced pluripotent stem cells derived from patients carrying disease causing mutations and thus established the first iPSC-based disease models.
• Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi:10.1126/science.1151526. In this seminal work, Yu and colleagues demonstrated that KLF4 and c-MYC could be replaced by LIN28 and NANOG in the reprogramming mix to efficiently derive human-induced pluripotent stem cells.
Siller R, Greenhough S, In-Hyun P, Sullivan G. Modelling human disease with pluripotent stem cells. Curr Gene Ther. 2013;13:99–110.
Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–4. doi:10.1016/j.stem.2009.04.005.
Fusaki N, Ban H, Nishiyama A, et al. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–62.
Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–30. doi:10.1016/j.stem.2010.08.012.
Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–12. doi:10.1038/nmeth.1591.
Zhao T, Zhang Z-N, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5. doi:10.1038/nature10135.
Guha P, Morgan J, Mostoslavsky G, et al. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12:407–12. doi:10.1016/j.stem.2013.01.006.
Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–4. doi:10.1038/nature11807.
Ware BR, Berger DR, Khetani SR. Prediction of drug-induced liver injury in micropatterned co-cultures containing iPSC-derived human hepatocytes. Toxicol Sci. 2015;145:252–62. doi:10.1093/toxsci/kfv048.
Guguen-Guillouzo C, Guillouzo A. General review on in vitro hepatocyte models and their applications. Methods Mol Biol. 2010;640:1–40. doi:10.1007/978-1-60761-688-7_1.
Gramignoli R, Tahan V, Dorko K, et al. New potential cell source for hepatocyte transplantation: discarded livers from metabolic disease liver transplants. Stem Cell Res. 2013;11:563–73. doi:10.1016/j.scr.2013.03.002.
Lu C, Li AP. Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague-Dawley rat, minipig, and beagle dog. Chem Biol Interact. 2001;134:271–81.
Zorn AM (2008) Liver development. StemBook. doi:10.3824/STEMBOOK.1.25.1.
Teo AKK, Ali Y, Wong KY, et al. Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells. 2012;30:631–42. doi:10.1002/stem.1022.
Jones EA, Clement-Jones M, James OF, Wilson DI. Differences between human and mouse alpha-fetoprotein expression during early development. J Anat. 2001;198:555–9. doi:10.1046/j.1469-7580.2001.19850555.x.
Bossard P, Zaret KS. GATA transcription factors as potentiators of gut endoderm differentiation. Development. 1998;125:4909–17.
Lokmane L, Haumaitre C, Garcia-Villalba P, et al. Crucial role of vHNF1 in vertebrate hepatic specification. Development. 2008;135:2777–86. doi:10.1242/dev.023010.
McPherson CE, Shim E-Y, Friedman DS, Zaret KS. An active tissue-specific enhancer and bound transcription factors existing in a precisely positioned nucleosomal array. Cell. 1993;75:387–98. doi:10.1016/0092-8674(93)80079-T.
Chaya D, Hayamizu T, Bustin M, Zaret KS. Transcription factor FoxA (HNF3) on a nucleosome at an enhancer complex in liver chromatin. J Biol Chem. 2001;276:44385–9. doi:10.1074/jbc.M108214200.
Cirillo LA, Lin FR, Cuesta I, et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9:279–89.
Buurman R, Gürlevik E, Schäffer V, et al. Histone deacetylases activate hepatocyte growth factor signaling by repressing microRNA-449 in hepatocellular carcinoma cells. Gastroenterology. 2012;143:811–820.e15. doi:10.1053/j.gastro.2012.05.033.
Bhattacharyya S, Tian J, Bouhassira EE, Locker J. Systematic targeted integration to study albumin gene control elements. PLoS One. 2011;6:e23234. doi:10.1371/journal.pone.0023234.
Calmont A, Wandzioch E, Tremblay KD, et al. An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev Cell. 2006;11:339–48. doi:10.1016/j.devcel.2006.06.015.
Twaroski K, Mallanna SK, Jing R, et al. FGF2 mediates hepatic progenitor cell formation during human pluripotent stem cell differentiation by inducing the WNT antagonist NKD1. Genes Dev. 2015;29:2463–74. doi:10.1101/gad.268961.115.
Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–63. doi:10.1126/science.1063889.
Margagliotti S, Clotman F, Pierreux CE, et al. Role of metalloproteinases at the onset of liver development. Develop Growth Differ. 2008;50:331–8. doi:10.1111/j.1440-169X.2008.01031.x.
Sosa-pineda B, Wigle JT. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25:254–5.
Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–89. doi:10.1016/j.devcel.2010.01.011.
Jochheim A, Hillemann T, Kania G, et al. Quantitative gene expression profiling reveals a fetal hepatic phenotype of murine ES-derived hepatocytes. Int J Dev Biol. 2004;48:23–9. doi:10.1387/IJDB.15005571.
Kelley-Loughnane N, Sabla GE, Ley-Ebert C, et al. Independent and overlapping transcriptional activation during liver development and regeneration in mice. Hepatology. 2002;35:525–34. doi:10.1053/jhep.2002.31351.
Petkov PM, Zavadil J, Goetz D, et al. Gene expression pattern in hepatic stem/progenitor cells during rat fetal development using complementary DNA microarrays. Hepatology. 2004;39:617–27. doi:10.1002/hep.20088.
Kyrmizi I, Hatzis P, Katrakili N, et al. Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev. 2006;20:2293–305. doi:10.1101/gad.390906.
Cui W, Sun M, Galeva N, et al. SUMOylation and ubiquitylation circuitry controls pregnane X receptor biology in hepatocytes. Drug Metab Dispos. 2015;43:1316–25. doi:10.1124/dmd.115.065201.
Imamura T, Cui L, Teng R, et al. Embryonic stem cell-derived embryoid bodies in three-dimensional culture system form hepatocyte-like cells in vitro and in vivo. Tissue Eng. 10:1716–24. doi:10.1089/ten.2004.10.1716.
Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol. 2006;50:645–52. doi:10.1387/ijdb.052072hb.
Basma H, Soto-Gutiérrez A, Yannam GR, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–9. doi:10.1053/j.gastro.2008.10.047.
Rambhatla L, Chiu C-P, Kundu P, et al. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11.
Hay DC, Zhao D, Ross A, et al. Direct differentiation of human embryonic stem cells to hepatocyte-like cells exhibiting functional activities. Cloning Stem Cells. 2007;9:51–62. doi:10.1089/clo.2006.0045.
D’Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–41. doi:10.1038/nbt1163.
Cai J, Zhao Y, Liu Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39. doi:10.1002/hep.21582.
Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–27. doi:10.1634/stemcells.2007-1102.
Brolén G, Sivertsson L, Björquist P, et al. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol. 2010;145:284–94. doi:10.1016/j.jbiotec.2009.11.007.
Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21. doi:10.1016/j.ydbio.2004.08.031.
Conlon F, Lyons K, Takaesu N, et al. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–28.
Zhou X, Sasaki H, Lowe L, et al. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–7. doi:10.1038/361543a0.
Jones C, Kuehn M, Hogan B, et al. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–62.
Feldman B, Gates MA, Egan ES, et al. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–5. doi:10.1038/26013.
Engert S, Burtscher I, Liao WP, et al. Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development. 2013;140:3128–38. doi:10.1242/dev.088765.
Loh KM, Ang LT, Zhang J, et al. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell. 2014;14:237–52. doi:10.1016/j.stem.2013.12.007.
Borowiak M, Maehr R, Chen S, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–58. doi:10.1016/j.stem.2009.01.014.
Tahamtani Y, Azarnia M, Farrokhi A, et al. Stauprimide priming of human embryonic stem cells toward definitive endoderm. Cell J. 2014;16:63–72.
Vallier L, Touboul T, Chng Z, et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS One. 2009;4:e6082. doi:10.1371/journal.pone.0006082.
Vallier L, Touboul T, Brown S, et al. Signaling pathways controlling pluripotency and early cell fate decisions of human induced pluripotent stem cells. Stem Cells. 2009;27:2655–66. doi:10.1002/stem.199.
Tahamtani Y, Azarnia M, Farrokhi A, et al. Treatment of human embryonic stem cells with different combinations of priming and inducing factors toward definitive endoderm. Stem Cells Dev. 2013;22:1419–32. doi:10.1089/scd.2012.0453.
Zhou J, Su P, Wang L, et al. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc Natl Acad Sci. 2009;106:7840–5. doi:10.1073/pnas.0901854106.
Ninomiya H, Mizuno K, Terada R, et al. Improved efficiency of definitive endoderm induction from human induced pluripotent stem cells in feeder and serum-free culture system. In Vitro Cell Dev Biol Anim. 2015;51:1–8. doi:10.1007/s11626-014-9801-y.
Chetty S, Pagliuca FW, Honore C, et al. A simple tool to improve pluripotent stem cell differentiation. Nat Methods. 2013;10:553–6. doi:10.1038/nmeth.2442.
Czysz K, Minger S, Thomas N. DMSO efficiently down regulates pluripotency genes in human embryonic stem cells during definitive endoderm derivation and increases the proficiency of hepatic differentiation. PLoS One. 2015;10:e0117689. doi:10.1371/journal.pone.0117689.
Maldonado M, Luu RJ, Ramos MEP, Nam J. ROCK inhibitor primes human induced pluripotent stem cells to selectively differentiate towards mesendodermal lineage via epithelial-mesenchymal transition-like modulation. Stem Cell Res. 2016; doi:10.1016/j.scr.2016.07.009.
Kondo Y, Iwao T, Yoshihashi S, et al. Histone deacetylase inhibitor valproic acid promotes the differentiation of human induced pluripotent stem cells into hepatocyte-like cells. PLoS One. 2014;9:e104010. doi:10.1371/journal.pone.0104010.
Touboul T, Chen S, To CC, et al. Stage-specific regulation of the WNT/β-catenin pathway enhances differentiation of hESCs into hepatocytes. J Hepatol. 2016;64:1315–26. doi:10.1016/j.jhep.2016.02.028.
Tasnim F, Phan D, Toh Y-C, Yu H. Cost-effective differentiation of hepatocyte-like cells from human pluripotent stem cells using small molecules. Biomaterials. 2015;70:115–25. doi:10.1016/j.biomaterials.2015.08.002.
Brown S, Teo A, Pauklin S, et al. Activin/Nodal signaling controls divergent transcriptional networks in human embryonic stem cells and in endoderm progenitors. Stem Cells. 2011;29:1176–85. doi:10.1002/stem.666.
Pauklin S, Vallier L. Activin/Nodal signalling in stem cells. Development. 2015;142:607–19. doi:10.1242/dev.091769.
Soto-Gutierrez A, Navarro-Alvarez N, Rivas-Carrillo JD, et al. Differentiation of human embryonic stem cells to hepatocytes using deleted variant of HGF and poly-amino-urethane-coated nonwoven polytetrafluoroethylene fabric. Cell Transplant. 2006;15:335–41.
Leiter JM, Helliger W, Puschendorf B. Increase in histone acetylation and transitions in histone variants during friend cell differentiation. Exp Cell Res. 1984;155:222–31.
McCoy AT, Benoist CC, Wright JW, et al. Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents. J Pharmacol Exp Ther. 2013;344:141–54. doi:10.1124/jpet.112.199497.
• Baxter M, Withey S, Harrison S, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62:581–9. doi:10.1016/j.jhep.2014.10.016. This paper from Baxter and colleagues establishes the critical aspects which should be addressed and assessed for all hPSC-derived hepatocyte-like ells in terms of maturity and function, thus providing a benchmark for which all subsequent reports should strive towards.
Patterson M, Chan DN, Ha I, et al. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2012;22:178–93. doi:10.1038/cr.2011.133.
Shan J, Schwartz RE, Ross NT, et al. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol. 2013;9:514–20. doi:10.1038/nchembio.1270.
• Avior Y, Levy G, Zimerman M, et al. Microbial-derived lithocholic acid and vitamin K 2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology. 2015;62:265–78. doi:10.1002/hep.27803. In this paper, Avior and colleagues demonstrated the maturation of hPSC-derived hepatocyte-like cells by using a combination of lithocholic acid and vitamin K, based on an analysis of postpartum changes in the environment of the developing liver.
Sun J, Mustafi R, Cerda S, et al. Lithocholic acid down-regulation of NF-kappaB activity through vitamin D receptor in colonic cancer cells. J Steroid Biochem Mol Biol. 2008;111:37–40. doi:10.1016/j.jsbmb.2008.01.003.
Cavin LG, Venkatraman M, Factor VM, et al. Regulation of -fetoprotein by nuclear factor- B protects hepatocytes from tumor necrosis factor- cytotoxicity during fetal liver development and hepatic oncogenesis. Cancer Res. 2004;64:7030–8. doi:10.1158/0008-5472.CAN-04-1647.
Zordoky BNM, El-Kadi AOS. Modulation of cardiac and hepatic cytochrome P450 enzymes during heart failure. Curr Drug Metab. 2008;9:122–8.
Ogawa S, Surapisitchat J, Virtanen C, et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development. 2013;140:3285–96. doi:10.1242/dev.090266.
Nakamori D, Takayama K, Nagamoto Y, et al. Hepatic maturation of human iPS cell-derived hepatocyte-like cells by ATF5, c/EBPα, and PROX1 transduction. Biochem Biophys Res Commun. 2016; doi:10.1016/j.bbrc.2015.12.007.
Gieseck RL III, Hannan NRF, Bort R, et al. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One. 2014;9:e86372. doi:10.1371/journal.pone.0086372.
• Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–4. doi:10.1038/nature12271. In this ground-breaking work from Takebe and colleagues, the generated functional liver buds through the combination of mesenchymal cells, endothelial cells, and hPSC-derived hepatic endoderm, which were highly functional and could efficiently engraft and be vascularized by a host mouse.
Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–4. doi:10.1126/science.1161431.
Asai A, Aihara E, Watson C, et al. Paracrine signals regulate human liver organoid maturation from iPSC. Development. 2017;144:142794. doi:10.1242/dev.142794.
Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–84. doi:10.1016/j.stem.2014.01.003.
Du Y, Wang J, Jia J, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell. 2014;14:394–403. doi:10.1016/j.stem.2014.01.008.
Huang P, He Z, Ji S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–9. doi:10.1038/nature10116.
Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–3. doi:10.1038/nature10263.
Cameron K, Tan R, Schmidt-Heck W, et al. Recombinant laminins drive the differentiation and self-organization of hESC-derived hepatocytes. Stem Cell Rep. 2015;5:1250–62. doi:10.1016/j.stemcr.2015.10.016.
Iredale JP, Arthur MJ. Hepatocyte-matrix interactions. Gut. 1994;35:729–32.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi:10.1016/j.cell.2006.06.044.
Dalby MJ, Biggs MJP, Gadegaard N, et al. Nanotopographical stimulation of mechanotransduction and changes in interphase centromere positioning. J Cell Biochem. 2007;100:326–38. doi:10.1002/jcb.21058.
Tung Y-C, Hsiao AY, Allen SG, et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–8. doi:10.1039/C0AN00609B.
Kempf H, Olmer R, Haase A, et al. Bulk cell density and Wnt/TGFbeta signalling regulate mesendodermal patterning of human pluripotent stem cells. Nat Commun. 2016;7:13602. doi:10.1038/ncomms13602.
Zweigerdt R, Olmer R, Singh H, et al. Scalable expansion of human pluripotent stem cells in suspension culture. Nat Protoc. 2011;6:689–700. doi:10.1038/nprot.2011.318.
Kempf H, Kropp C, Olmer R, et al. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat Protoc. 2015;10:1345–61. doi:10.1038/nprot.2015.089.
Kempf H, Olmer R, Kropp C, et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 2014;3:1132–46. doi:10.1016/j.stemcr.2014.09.017.
Olmer R, Lange A, Selzer S, et al. Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Tissue Eng Part C Methods. 2012;18:772–84. doi:10.1089/ten.TEC.2011.0717.
Paull D, Sevilla A, Zhou H, et al. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat Methods. 2015;12:885–92. doi:10.1038/nmeth.3507.
Chen KG, Mallon BS, McKay RDG, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell. 2014;14:13–26. doi:10.1016/j.stem.2013.12.005.
Imaizumi K, Sone T, Ibata K, et al. Controlling the regional identity of hPSC-derived neurons to uncover neuronal subtype specificity of neurological disease phenotypes. Stem Cell Rep. 2015;5:1010–22. doi:10.1016/j.stemcr.2015.10.005.
Denning C, Borgdorff V, Crutchley J, et al. Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim Biophys Acta Mol Cell Res. 2016;1863:1728–48. doi:10.1016/j.bbamcr.2015.10.014.
Colnot S, Perret C. Liver zonation. US: Springer; 2011. p. 7–16.
Gebhardt R, Matz-Soja M. Liver zonation: novel aspects of its regulation and its impact on homeostasis. World J Gastroenterol. 2014;20:8491–504. doi:10.3748/wjg.v20.i26.8491.
Orloff J, Douglas F, Pinheiro J, et al. The future of drug development: advancing clinical trial design. Nat Rev Drug Discov. 2009;8:949. doi:10.1038/nrd3025.
Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–85. doi:10.1056/NEJMra021844.
Kimbrel EA, Lanza R. Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov. 2015;14:681–92. doi:10.1038/nrd4738.
Sharma R, Greenhough S, Medine CN, Hay DC. Three-dimensional culture of human embryonic stem cell derived hepatic endoderm and its role in bioartificial liver construction. J Biomed Biotechnol. 2010;2010:236147. doi:10.1155/2010/236147.
Acknowledgements
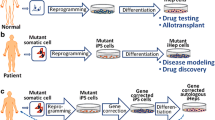
The authors would like to thank Carina Knudsen at the University of Oslo Photo and Graphics Service for the illustration in Fig. 1.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Richard Siller, Santosh Mathapati, and Karim Si-Tayeb declare that they have no conflict of interest.
Gareth J Sullivan is named as an inventor on a provisional application for a patent describing the small molecule-driven hepatocyte differentiation of human pluripotent stem cells.
Sebastian Greenhough is named as an inventor on a provisional application for a patent describing the small molecule-driven hepatocyte differentiation of human pluripotent stem cells.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Application of Stem Cells in Endoderm Derivatives
Rights and permissions
About this article
Cite this article
Siller, R., Greenhough, S., Mathapati, S. et al. Future Challenges in the Generation of Hepatocyte-Like Cells From Human Pluripotent Stem Cells. Curr Pathobiol Rep 5, 301–314 (2017). https://doi.org/10.1007/s40139-017-0150-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-017-0150-x