Abstract
Purpose of Review
Epithelial cells line tissues and serve an important functional role in maintaining a barrier between tissue compartments and protection from the external environment. Tight Junctions and apical–basal polarity function to establish and maintain epithelial structures to prevent leakage between cells and define distinct subcellular domains to localize signaling pathways within cells that control growth, survival, and migration. Disruption of tight junctions and cell polarity is frequently observed in cancer.
Recent Findings
In this review, we discuss a role for Tight Junctions and the Par polarity complex as a sensor of epithelial integrity that contributes to maintaining epithelial homeostasis through apoptosis, proliferation, and stem cell renewal, and that disruption of these processes contributes to cancer progression.
Summary
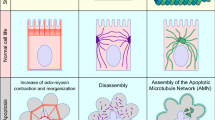
Epithelial homeostasis is maintained by the spatial organization of signaling pathways within cells. Damaged cells are eliminated from the tissue by extrusion and apoptosis, and are balanced by proliferation and differentiation. Understanding the control of these processes during normal tissue development and homeostasis will lead to a more complete view of the etiology of cancer.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Van Itallie CM, Anderson JM (2014) Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 36:157–165
Zihni C, Balda MS, Matter K (2014) Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J Cell Sci 127(Pt 16):3401–3413
Citi S et al (2014) Epithelial junctions and Rho family GTPases: the zonular signalosome. Small GTPases 5(4):1–15
Rodriguez-Boulan E, Macara IG (2014) Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15(4):225–242
Chen J, Zhang M (2013) The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res 319(10):1357–1364
Elsum I et al (2012) The Scribble-Dlg-Lgl polarity module in development and cancer: from flies to man. Essays Biochem 53:141–168
Goehring NW (2014) PAR polarity: from complexity to design principles. Exp Cell Res 328(2):258–266
Smith CA et al (2007) aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J 26(2):468–480
Diaz-Meco MT, Moscat J (2012) The atypical PKCs in inflammation: NF-kappaB and beyond. Immunol Rev 246(1):154–167
Momcilovic M, Shackelford DB (2015) Targeting LKB1 in cancer— exposing and exploiting vulnerabilities. Br J Cancer 113(4):574–584
Godde NJ et al (2014) Dissecting the role of polarity regulators in cancer through the use of mouse models. Exp Cell Res 328(2):249–257
Chatterjee SJ, McCaffrey L (2014) Emerging role of cell polarity proteins in breast cancer progression and metastasis. Breast Cancer 6:15–27
Halaoui R, McCaffrey L (2015) Rewiring cell polarity signaling in cancer. Oncogene 34(8):939–950
Hinck L, Nathke I (2014) Changes in cell and tissue organization in cancer of the breast and colon. Curr Opin Cell Biol 26:87–95
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196
Viloria-Petit AM, Wrana JL (2010) The TGFbeta-Par6 polarity pathway: linking the Par complex to EMT and breast cancer progression. Cell Cycle 9(4):623–624
Sanchez NS, Barnett JV (2012) TGFbeta and BMP-2 regulate epicardial cell invasion via TGFbetaR3 activation of the Par6/Smurf1/RhoA pathway. Cell Signal 24(2):539–548
Viloria-Petit AM et al (2009) A role for the TGFbeta-Par6 polarity pathway in breast cancer progression. Proc Natl Acad Sci USA 106(33):14028–14033
Ozdamar B et al (2005) Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307(5715):1603–1609
Suzanne M, Steller H (2013) Shaping organisms with apoptosis. Cell Death Differ 20(5):669–675
Green DR, Llambi F (2015) Cell Death Signaling. Cold Spring Harb Perspect Biol 7(12):a006080
Williams JM et al (2015) Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet Pathol 52(3):445–455
Guan Y et al (2011) Redistribution of the Tight Junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am J Physiol Cell Physiol 300(6):C1404–C1414
Bullen TF et al (2006) Characterization of epithelial cell shedding from human small intestine. Lab Invest 86(10):1052–1063
Mailleux AA et al (2007) BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell 12(2):221–234
Stelwagen K, Singh K (2014) The role of Tight Junctions in mammary gland function. J Mammary Gland Biol Neoplasia 19(1):131–138
Colitti M (2012) BCL-2 family of proteins and mammary cellular fate. Anat Histol Embryol 41(4):237–247
Beeman NE et al (2009) Disruption of occludin function in polarized epithelial cells activates the extrinsic pathway of apoptosis leading to cell extrusion without loss of transepithelial resistance. BMC Cell Biol 10:85
Beeman N, Webb PG, Baumgartner HK (2012) Occludin is required for apoptosis when claudin–claudin interactions are disrupted. Cell Death Dis 3:e273
Durgan J et al (2011) Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J Biol Chem 286(14):12461–12474
Trani M et al (2009) Pro-apoptotic effect of aurothiomalate in prostate cancer cells. Cell Cycle 8(2):306–313
Avery-Cooper G et al (2014) Par6 is an essential mediator of apoptotic response to transforming growth factor beta in NMuMG immortalized mammary cells. Cancer Cell Int 14(1):19
Gunaratne A, Thai BL, Di Guglielmo GM (2013) A typical protein kinase C phosphorylates Par6 and facilitates transforming growth factor beta-induced epithelial-to-mesenchymal transition. Mol Cell Biol 33(5):874–886
Gunaratne A et al (2015) aPKC alters the TGFbeta response in NSCLC cells through both Smad-dependent and Smad-independent pathways. J Cell Sci 128(3):487–498
McCaffrey LM, Macara IG (2009) The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev 23(12):1450–1460
Iden S et al (2012) Tumor type-dependent function of the par3 polarity protein in skin tumorigenesis. Cancer Cell 22(3):389–403
Slattum G, McGee KM, Rosenblatt J (2009) P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J Cell Biol 186(5):693–702
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 1833(12):3481–3498
Eisenhoffer GT, Rosenblatt J (2013) Bringing balance by force: live cell extrusion controls epithelial cell numbers. Trends Cell Biol 23(4):185–192
Gu Y, Rosenblatt J (2012) New emerging roles for epithelial cell extrusion. Curr Opin Cell Biol 24(6):865–870
Gu Y et al (2015) Defective apical extrusion signaling contributes to aggressive tumor hallmarks. Elife 4:e04069
• Krawchuk D et al (2015) Loss of LKB1 leads to impaired epithelial integrity and cell extrusion in the early mouse embryo. J Cell Sci 128(5):1011–1022. This paper shows that loss of Lkb1 induces apical extrusion of epithelial cells that can survive anoikis
Guo X et al (2015) Par3 regulates invasion of pancreatic cancer cells via interaction with Tiam1. Clin Exp Med 1–9
Xue B et al (2013) Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat Cell Biol 15(2):189–200
McCaffrey LM et al (2012) Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 22(5):601–614
Zen K et al (2009) Defective expression of polarity protein PAR-3 gene (PARD3) in esophageal squamous cell carcinoma. Oncogene 28(32):2910–2918
• Archibald A et al (2015) Oncogenic suppression of apoptosis uncovers a Rac1/JNK proliferation pathway activated by loss of Par3. Oncogene 34(24):3199–3206. This paper demonstrates that loss of Par3 induces apoptosis, but in switches to enhance proliferation when apoptosis is blocked. This provides evidence for how cells that lose apical-basal polarity may contribute to tumor growth
Archibald A et al (2015) A typical protein kinase C induces cell transformation by disrupting Hippo/Yap signaling. Mol Biol Cell 26(20):3578–3595
Linch M et al (2014) Regulation of polarized morphogenesis by protein kinase C iota in oncogenic epithelial spheroids. Carcinogenesis 35(2):396–406
Meng Z, Moroishi T, Guan KL (2016) Mechanisms of Hippo pathway regulation. Genes Dev 30(1):1–17
Zhao B et al (2011) Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev 25(1):51–63
Varelas X et al (2010) The crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell 19(6):831–844
Cong W et al (2010) ASPP2 regulates epithelial cell polarity through the PAR complex. Curr Biol 20(15):1408–1414
• Royer C et al (2014) ASPP2 links the apical lateral polarity complex to the regulation of YAP activity in epithelial cells. PLoS One 9(10):e111384. This paper demonstrates that the Tight Junction protein ASPP2 is able to bind the phosphatase PP1 and dephosphorylate Yap1 to enable its translocation to the nucleus
•• Lv XB et al (2015) PARD3 induces TAZ activation and cell growth by promoting LATS1 and PP1 interaction. EMBO Rep 16(8):975–985. The authors demonstrate that Par3 can bind PP1 and Lats1 to dephosphorylate Lats1. Dephosphorylated Lats1 prevents Taz phosphorylation and releases it to the nucleus to induce proliferation. Interestingly, the interaction between Par3, PP1, and Lats1 only occurs in the cytoplasm, providing a mechanism by which loss of Tight Junctions release Par3 to inhibit the Hippo pathway and promote proliferation
Bergstralh DT, Haack T, St Johnston D (2013) Epithelial polarity and spindle orientation: intersecting pathways. Philos Trans R 368(1629):20130291
Williams SE, Fuchs E (2013) Oriented divisions, fate decisions. Curr Opin Cell Biol 25(6):749–758
Hao Y et al (2010) Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr Biol 20(20):1809–1818
•• Tuncay H et al (2015) JAM-A regulates cortical dynein localization through Cdc42 to control planar spindle orientation during mitosis. Nat Commun 6:8128. This article demonstrates how the Tight Junction molecule JAM-A regulates spindle orientation by controlling the anchoring of dynein to the cell membrane. They identify a phospholipid gradient and actin skeletal remodeling as key events
•• Niessen MT et al (2013) aPKC lambda controls epidermal homeostasis and stem cell fate through regulation of division orientation. J Cell Biol, 202(6):887–900. The authors demonstrate that atypical PKC lamda/iota controls spindle orientation and stem cell self-renewal in epidermal and bulge stem cells in the hair follicle
•• Huo Y, Macara IG (2014) The Par3-like polarity protein Par3L is essential for mammary stem cell maintenance. Nat Cell Biol 16(6):529–37. This paper identifies a role for a new polarity protein Par3-like (Par3L), in regulating stem cell maintenance in the mammary epithelium. Loss of Par3L causes depletion of multipotent mammary stem cells by regulating the activity of the tumor suppressor Lkb1
Acknowledgments
We apologize to colleagues whose original work was not cited because of scope and space limitations. SJC is supported by a McGill Integrated Cancer Research Training Program (MICRTP) graduate studentship. RH is supported by a graduate studentship from FRQS and LM is a FRQS Scholar (Junior I). LM is funded by grants from the Cancer Research Society, Quebec Breast Cancer Foundation, and Canadian Institutes of Health Research. We also thank members of the McCaffrey Laboratory for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sudipa June Chatterjee, Ruba Halaoui, and Luke McCaffrey declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
For articles cited in references for which one of the authors was also an author, we confirm that studies were performed in accordance with protocols that were approved by Institutional Animal Review Committees.
Additional information
Sudipa June Chatterjee and Ruba Halaoui have equally contributed to this work.
This article is part of the Topical collection on Leaky Junctions in Cancer.
Rights and permissions
About this article
Cite this article
Chatterjee, S.J., Halaoui, R. & McCaffrey, L. Apical–Basal Polarity as a Sensor for Epithelial Homeostasis: A Matter of Life and Death. Curr Pathobiol Rep 4, 99–106 (2016). https://doi.org/10.1007/s40139-016-0107-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-016-0107-5