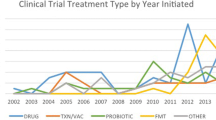
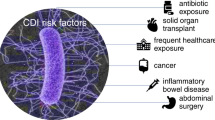
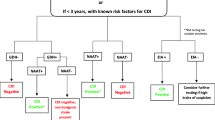
Abstract
Purpose of Review
The goal of this review was to determine the current treatment and prevention recommendations for Clostridium difficile infections (CDI) and their efficacy. We elucidate new treatment and prevention strategies that are in development, and define populations that are at greatest risk of CDI.
Recent Findings
New practice guidelines recommend vancomycin or fidaxomicin as first-line treatment for CDI with oral metronidazole only being used in cases of non-severe CDI when vancomycin and fidaxomicin are not available. Metronidazole is recommended in an IV formulation in conjunction with oral vancomycin in fulminant CDI. Recurrent CDI (rCDI) may be treated with vancomycin, fidaxomicin, or fecal microbiota transplant (FMT). Recent studies show fidaxomicin and fecal microbiota transplantation to be the most effective in decreasing the risk of rCDI. Prevention of CDI is primarily through judicious use of antibiotics and strategies aimed at minimizing the spread of C. difficile spores in hospitals. New preventative options are being studied including an oral beta-lactamase that may decrease the risk of developing CDI after beta-lactam use and a potential vaccine against toxins A and B produced by C. difficile.
Summary
rCDI continues to be a significant problem particularly in older populations. New treatment guidelines may provide increased protection of recurrence as new medications and treatment modalities are more often utilized. Adjunctive treatments currently being studied may provide additional protection against recurrence but prevention against initial (iCDI) is still of great importance.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ma G, Brensinger C, Wu Q, Lewis J. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States. Ann Intern Med. 2017;167:152–8.
Lessa F, Mu Y, Bamberg W. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–34.
Hall A, Curns A, McDonald L, Parashar U, Lopman B. The roles of Clostridium difficile and Norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55:216–23.
Freeman J, Bauer M, Baines S, Corver J, Fawley W, Goorhuis B, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–49.
Chitnis A, Holzbauer S, Belflower R, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 Through 2011. JAMA Intern Med. 2013;173:1359–67.
Khanna S, Pardi D, Aronson S, Kammer P, Orenstein R, St Sauver J, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95.
Dubberke E, Olsen M. burden of clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55:S88–92.
Just I, Wilm M, Selzer J, Rex G, von Eichel-Streiber C, Mann M, et al. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995;270:13932–6.
Ohshima T, Osaki T, Yamamoto Y, Asai S, Miyachi H, Kamiya S. Evaluation of risk factors for Clostridium difficile infection based on immunochromatography testing and toxigenic culture assay. J Clin Microbiol. 2018;56. https://doi.org/10.1128/jcm.00555-18.
Adams D, Eberly M, Rajnik M, Nylund C. Risk factors for community-associated Clostridium difficile infection in children. J Pediatr. 2017;186:105–9.
Sammons J, Toltzis P, Zaoutis T. Clostridium difficile infection in children. JAMA Pediatr. 2013;167:567–73.
Faden H, Ma C. Trends in oral antibiotic, proton pump inhibitor, and histamine 2 receptor blocker prescription patterns for children compared with adults. Clin Pediatr. 2015;55:712–6.
Negrón M, Rezaie A, Barkema H, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study. Am J Gastroenterol. 2016;111:691–704.
Peng J, Shen J, Zhu Q, Ran Z. The impact of Clostridium difficile on surgical rate among ulcerative colitis patients: a systemic review and meta-analysis. Saudi J Gastroenterol. 2015;21:208.
Razik R, Rumman A, Bahreini Z, McGeer A, Nguyen G. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the RECIDIVISM study. Am J Gastroenterol. 2016;111:1141–6.
Olsen M, Yan Y, Reske K, Zilberberg M, Dubberke E. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect. 2015;21:164–70.
Rodriguez C, Korsak N, Taminiau B, Avesani V, Van Broeck J, Delmée M, et al. Clostridium difficile infection in elderly nursing home residents. Anaerobe. 2014;30:184–7.
Zilberberg M, Reske K, Olsen M, Yan Y, Dubberke E. Risk factors for recurrent Clostridium difficile infection (CDI) hospitalization among hospitalized patients with an initial CDI episode: a retrospective cohort study. BMC Infect Dis. 2014;14:306.
Garey K, Sethi S, Yadav Y, DuPont H. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298–304.
Fekety R, McFarland L, Surawicz C, Greenberg R, Elmer G, Mulligan M. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324–33.
McFarland L, Surawicz C, Rubin M, Fekety R, Elmer G, Greenberg R. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20:43–50.
Cadle R, Mansouri M, Logan N, Kudva D, Musher D. Association of proton-pump inhibitors with outcomes in Clostridium difficile colitis. Am J Health Syst Pharm. 2007;64:2359–63.
Kim J, Lee K, Jeong J, Kim B, Shin S, Kim J, et al. Proton pump inhibitors as a risk factor for recurrence of Clostridium-difficile-associated diarrhea. World J Gastroenterol. 2010;16:3573–677.
Donnelly J, Wang H, Locke J, Mannon R, Safford M, Baddley J. Hospital-onset Clostridium difficile infection among solid organ transplant recipients. Am J Transplant. 2015;15:2970–7.
Paudel S, Zacharioudakis I, Zervou F, Ziakas P, Mylonakis E. Prevalence of Clostridium difficile infection among solid organ transplant recipients: a meta-analysis of published studies. PLoS One. 2015;10:e0124483.
Zacharioudakis I, Ziakas P, Mylonakis E. Clostridium difficile infection in the hematopoietic unit: a meta-analysis of published studies. Biol Blood Marrow Transplant. 2014;20:1641–65.
•• McDonald L, Gerding D, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:e1–e48 This reference was important in providing current guidelines for the treatment and prevention of CDI.
Centers for Disease Control and Prevention. Emerging infections program healthcare-associated infections projects. 2015. Available at: http://www.cdc.gov/hai/eip/index.html. Accessed 9 March 2016.
Bobulsky G, Al-Nassir W, Riggs M, Sethi A, Donskey C. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis. 2008;46:447–50.
Curry S, Muto C, Schlackman J, Pasculle A, Shutt K, Marsh J, et al. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin Infect Dis. 2013;57:1094–102.
Johnson S, Gerding D, Olson M, Weiler M, Hughes R, Clabots C, et al. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission. Am J Med. 1990;88:137–40.
Gordin F, Schultz M, Huber R, Gill J. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infect Control Hosp Epidemiol. 2005;26:650–3.
Dubberke E, Carling P, Carrico R, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:628–45.
Vajravelu R, Guerrero D, Jury L, Donskey C. Evaluation of stethoscopes as vectors of Clostridium difficile and methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2012;33:96–8.
Manian F, Meyer L, Jenne J. Clostridium difficile contamination of blood pressure cuffs: a call for a closer look at gloving practices in the era of universal precautions. Infect Control Hosp Epidemiol. 1996;17:180–2.
Jernigan J, Siegman-Igra Y, Guerrant R, Farr B. A randomized crossover study of disposable thermometers for prevention of Clostridium difficile and other nosocomial infections. Infect Control Hosp Epidemiol. 1998;19:494–9.
Sears P, Crook D, Louie T, Miller M, Weiss K. Fidaxomicin attains high fecal concentrations with minimal plasma concentrations following oral administration in patients with Clostridium difficile infection. Clin Infect Dis. 2012;55:S116–20.
Oshima H, Yamazaki T, Benner L, Miki T, Michon I, Wojtkowski T, et al. Comparison of the safety, tolerability, and pharmacokinetics of fidaxomicin in healthy Japanese and Caucasian subjects. Clin Drug Investig. 2015;35:375–84.
Nerandzic M, Mullane K, Miller M, Babakhani F, Donskey C. Reduced acquisition and overgrowth of vancomycin-resistant Enterococci and Candida species in Patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin Infect Dis. 2012;55:S121–6.
Slimings C, Riley T. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:881–91.
Mullane K, Miller M, Weiss K, Lentnek A, Golan Y, Sears P, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. 2011;53:440–7.
Rao K, Micic D, Natarajan M, Winters S, Kiel MJ, Walk ST, et al. Clostridium difficile ribotype 027: relationship to age, detectability of toxins A or B in stool with rapid testing, severe infection, and mortality. Clin Infect Dis. 2015;61:233–41.
Abou Chakra C, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9:e98400.
Zhanel G, Walkty A, Karlowsky J. Fidaxomycin: a novel agent for the treatment of Clostridium difficile. Canadian Journal of Infectious Disease Medical Microbiology. 2015;26:305–12.
Goldstein E, Babakhani F, Citron D. Antimicrobial activities of fidaxomicin. Clin Infect Dis. 2012;55:S143–8.
Gerber M, Ackermann G. OPT-80, a macrocyclic antimicrobial agent for the treatment of Clostridium difficile infections: a review. Expert Opin Investig Drugs. 2008;17:547–53.
Tannock G, Munro K, Taylor C, Lawley B, Young W, Byrne B, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156:3354–9.
Chilton C, Crowther G, Freeman J, Todhunter S, Nicholson S, Longshaw C, et al. Successful treatment of simulated Clostridium difficile infection in a human gut model by fidaxomicin first line and after vancomycin or metronidazole failure. J Antimicrob Chemother. 2013;69:451–62.
Louie T, Cannon K, Byrne B, Emery J, Ward L, Eyben M, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;55:S132–42.
Louie T, Miller M, Mullane K, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31.
• Al Momani L, Abughanimeh O, Boonpherg B, Gabriel J, Young M. Fidaxomicin vs vancomycin for the treatment of a first episode of Clostridium Difficile infection: a meta-analysis and systematic review. Cureus. 2018. https://doi.org/10.7759/cureus.2778 This reference was important in comparing the efficacy of vancomycin and fidaxomicin for the treatment of CDI.
Babakhani F, Gomez A, Robert N, Sears P. Killing kinetics of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. J Med Microbiol. 2011;60:1213–7.
Cruz M. Fidaxomicin (Dificid), a novel oral macrocyclic antibacterial agent for the treatment of Clostridium difficile – associated diarrhea in adults. Pharm Ther. 2012;37:478–281.
Gallagher J, Reilly J, Navalkele B, Downham G, Haynes K, Trivedi M. Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Antimicrob Agents Chemother. 2015;59:7007–10.
Reveles K, Backo J, Corvino F, Zivkovic M, Broderick K. Fidaxomicin versus vancomycin as a first-line treatment for Clostridium difficile-associated diarrhea in specific patient populations: a pharmacoeconomic evaluation. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2017;37:1489–97.
Le P, Nghiem V, Mullen P, Deshpande A. Cost-effectiveness of competing treatment strategies for Clostridium difficile infection: a systematic review. Infect Control Hosp Epidemiol. 2018;39:412–24.
Khoruts A, Dicksved J, Jansson J, Sadowsky M. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–60.
Sorg J, Sonenshein A. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J Bacteriol. 2009;191:1115–7.
Ridlon J, Harris S, Bhowmik S, Kang D, Hylemon P. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39.
Thanissery R, Winston J, Theriot C. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe. 2017;45:86–100.
Rossen N, MacDonald J, de Vries E, et al. Fecal microbiota transplantation as novel therapy in gastroenterology: a systematic review. World J Gastroenterol. 2015;21:5359–71.
Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection. J Clin Gastroenterol. 2014;48:693–702.
Kassam Z, Lee C, Yuan Y, Hunt R. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–8.
Mamo Y, Woodworth M, Wang T, Dhere T, Kraft C. Durability and long-term clinical outcomes of fecal microbiota transplant treatment in patients with recurrent Clostridium difficile infection. Clin Infect Dis. 2017;66:1705–11.
Girotra M, Garg S, Anand R, Song Y, Dutta S. Fecal microbiota transplantation for recurrent Clostridium difficile infection in the elderly: long-term outcomes and microbiota changes. Dig Dis Sci. 2016;61:3007–15.
Minino A, Xu J, Kochanek K. Deaths: preliminary data for 2008; 2010. p. 1–52.
Cornely O, Crook D, Esposito R, Poirier A, Somero M, Weiss K, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–9.
Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston D, Hernandez A, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452–60.
Guery B, Menichetti F, Anttila V, Adomakoh N, Aguado JM, Bisnauthsing K, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomized, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis. 2018;18:296–307.
Johnson S, Schriever C, Patel U, Patel T, Hecht D, Gerding D. Rifaximin redux: treatment of recurrent Clostridium difficile infections with rifaximin immediately post-vancomycin treatment. Anaerobe. 2009;15:290–1.
Garey K, Ghantoji S, Shah D, Habib M, Arora V, Jiang Z, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhea in patients with Clostridium difficile infection. J Antimicrob Chemother. 2011;66:2850–5.
• Wilcox M, Gerding D, Poxton I, Kelly C, Nathan R, Birch T, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305–17 This reference was important in showing the efficacy of a newly FDA-approved treatment adjunct for CDI.
Zhang H, Morrison S, Tang Y. Multiplex polymerase chain reaction tests for detection of pathogens associated with gastroenteritis. Clin Lab Med. 2015;35:461–86.
Kokai-Kun J, Roberts T, Coughlin O, et al. The oral β-lactamase SYN-004 (ribaxamase) degrades ceftriaxone excreted into the intestine in phase 2a clinical studies. Antimicrob Agents Chemother. 2017;61. https://doi.org/10.1128/aac.02197-16.
Dubischar K, et al (2016) A phase 2, dose-confirmation immunogenicity and safety study of Vla84, a Clostridium difficile vaccine candidate, in adults aged 50 years and older (abstract). ASM Microbe
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Humans and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infectious Disease
Rights and permissions
About this article
Cite this article
Parmar, N.V., Glauser, J. Systematic Review of Current Treatment and Prevention Strategies for Clostridium difficile. Curr Emerg Hosp Med Rep 7, 66–73 (2019). https://doi.org/10.1007/s40138-019-00186-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-019-00186-1