Abstract
Purpose of Review
Hyperammonemia have significant morbidity and mortality. In this paper, we reviewed the latest research and evidence of both conventional and upcoming oral or intravenous treatments.
Recent Findings
New updates on the role of oral agents such as rifaximin, PEG, probiotics, glycerol phenylbutyrate, and zinc supplements in the management of both chronic hyperammonemia and overt hepatic encephalopathy have been discussed in this review. We discussed the recent findings on the role of branched-chain amino acids in patients with cirrhosis.
Summary
Rifaximin role has expanded as mono or combination therapy in patients with hepatic encephalopathy. Probiotics and zinc might play a role in the prevention of overt hepatic encephalopathy. Newer oral agents such as activated carbon seem to be promising. Saline or albumin might play a role in diuretic-induced hyperammonemia but their role is yet to be determined. More research is needed for new interventions and treatment validation.


Similar content being viewed by others
Abbreviations
- ASL:
-
Argininosuccinic acid lyase
- ASS:
-
Argininosuccinate synthetase
- ATP:
-
Adenosine 5′-triphosphate
- BBB:
-
Blood-brain barrier
- BCAA:
-
Branch chain amino acids
- CPS:
-
Carbamyl phosphate synthetase
- CT:
-
Computed tomography
- GI:
-
Gastrointestinal
- ICH:
-
Intracranial hemorrhage
- IEM:
-
Inborn error of metabolism
- IV:
-
Intravenous
- LOLA:
-
L-Ornithine-L-aspartate
- MRI:
-
Magnetic resonance imaging
- NAGS:
-
N-Acetyl glutamine synthetase
- OTC:
-
Ornithine transcarbamylase
- RRT:
-
Renal replacement therapy
- TPN:
-
Total parenteral nutrition
- UCDs:
-
Urea cycle disorders
- US:
-
Ultrasonography
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Bachmann C. Mechanisms of hyperammonemia. Clin Chem Lab Med. 2002;40:653–62.
Vince A, Dawson AM, Park N, O’Grady F. Ammonia production by intestinal bacteria. Gut. 1973;14:171–7.
Karim Z, Szutkowska M, Vernimmen C, et al. Renal handling of NH3/NH4+: recent concepts. Nephron Physiol. 2005;101:77–81.
Olde Damink SW, Dejong CH, Deutz NE, et al. Kidney plays a major role in ammonia homeostasis after portasystemic shunting in patients with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G189–94.
Ott P, Vilstrup H. Cerebral effects of ammonia in liver disease: current hypotheses. Metab Brain Dis. 2014;29:901–11.
Reeds P, Burrin D, Stoll C, et al. Intestinal glutamate metabolism. J Nutr. 2000;130(4):978S–82S.
Benque A, Bommelaer G, Rosental G. Chronic vomiting in a case of citrullinaemia detected after treatment by total parenteral nutrition. Gut. 1984;25:531–533 A.
Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132(4):1368–78.
Machado MC, Pinheiro da Silva F. Hyperammonemia due to urea cycle disorders: a potentially fatal condition in the intensive care setting. J Intensive Care. 2014;2(1):22.
Albrecht J, Zielinska M, Norenberg MD. Glutamine as a mediator of ammonia neurotoxicity: a critical appraisal. Biochem Pharmacol. 2010;80(9):1303–8.
Patil D, Westaby D, Mahida Y, et al. Comparative modes of action of lactitol and lactulose in the treatment of hepatic encephalopathy. Gut. 1987;28:255–9.
Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver Int. 2011;31:163–75.
Helman G, Pacheco-Colón I, Gropman A. The urea cycle disorders. Semin Neurol. 2014;34(3):341–9.
Brusilow SW, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, and therapy. Adv Pediatr Infect Dis. 1996;43:127–70.
Grisar T. Argininosuccinic aciduria in adult: a clinical, electrophysiological and biochemical study. Adv Exp Med Biol. 1982;153:83–93.
Kobayashi K, Shaheen N, Kumashiro R, Tanikawa K, O’Brien WE, Beaudet AL, et al. A search for the primary abnormality in adult-onset type II citrullinemia. Am J Hum Genet. 1993;53:1024–30.
Summar M, Barr F, Dawling S, et al. Unmasked adult-onset urea cycle disorders in the critical care setting. Crit Care Clin. 2005;21(suppl):S1–8.
Butterworth RF. Effects of hyperammonaemia on brain function. J Inherit Metab Dis. 1998;21:6–20.
Shawcross D, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62:2295–304.
Vaquero J, Chung C, Cahill ME, Blei AT. Pathogenesis of hepatic encephalopathy in acute liver failure. Semin Liver Dis. 2003;23:259–69.
Rangroo Thrane V, Thrane AS, Wang F, et al. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med. 2013;19(12):1643–8.
Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis. 2007;22(3–4):199–218.
Clemmesen JO, Larsen FS, Kondrup J, et al. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29(3):648–53.
Kulick SK, Kramer DA. Hyperammonemia secondary to valproic acid as a cause of lethargy in a postictal patient. Ann Emerg Med. 1993;22:610–2.
Ambrosetto G, Riva R, Baruzzi A. Hyperammonemia in asterixis induced by carbamazepine: two case reports. Acta Neurol Scand. 1984;69:186–9.
Bertrand P, Faro A, Cantwell P, Tzakis A. Intravenous ribavirin and hyperammonemia in an immunocompromised patient infected with adenovirus. Pharmacotherapy. 2000;20:1216–20.
Sekas G, Paul HS. Hyperammonemia and carnitine deficiency in a patient receiving sulfadiazine and pyrimethamine. Am J Med. 1993;95:112–3.
Ishikawa F, Nakamuta M, Kato M, et al. Reversibility of serum NH3 level in a case of sudden onset and rapidly progressive case of type 2 citrullinemia. Intern Med. 2000;39:925–9.
Solga S. Probiotics can treat hepatic encephalopathy. Med Hypotheses. 2003;61:307–13.
Samtoy B, DeBeukelaer MM. Ammonia encephalopathy secondary to urinary tract infection with Proteus mirabilis. Pediatrics. 1980;65:294–7.
Cheang HK, Rangecroft L, Plant ND, Morris AAM. Hyperammonaemia due to Klebsiella infection in a neuropathic bladder. Pediatr Nephrol. 1998;12:658–9.
Kaveggia FF, Thompson JS, Schafer EC, Fischer JL, Taylor RJ. Hyperammonemic encephalopathy in urinary diversion with urea-splitting urinary tract infection. Arch Intern Med. 1990;150:2389–92.
Nurmohamed S, Weenink A, Moeniralam H, et al. Hyperammonemia in generalized Mycobacterium genavense infection after renal transplantation. Am J Transplant. 2007;7(3):722–3.
Wylam ME, Kennedy CC, Hernandez NM. Fatal hyperammonaemia caused by Mycoplasma hominis. Lancet. 2013;382(9908):1956.
Barnes PM, Wheldon DB, Eggerding C, Marshall WC, Leonard JV. Hyperammonaemia and disseminated herpes simplex infection in the neonatal period. Lancet. 1982;1:1362–3.
Lo WD, Sloan HR, Sotos JF, Klinger RJ. Late clinical presentation of partial carbamyl phosphate synthetase I deficiency. Am J Dis Child. 1993;147:267–9.
Finkelstein JE, Hauser ER, Leonard CO, Brusilow SW. Late-onset ornithine transcarbamylase deficiency in male patients. J Pediatr. 1990;117:897–902.
Schimanski U, Krieger D, Horn M, Stremmel W, Wermuth B, Theilmann L. A novel two-nucleotide deletion in the ornithine transcarbamylase gene causing fatal hyperammonia in early pregnancy. Hepatology. 1996;24:1413–5.
Arn PH, Hauser ER, Thomas GH, Herman G, Hess D, Brusilow SW. Hyperammonemia in women with a mutation at the ornithine carbamoyltransferase locus: a cause of postpartum coma. N Engl J Med. 1990;322:1652–5.
Felig DM, Brusilow SW, Boyer JL. Hyperammonemic coma due to parenteral nutrition in a woman with heterozygous ornithine transcarbamylase deficiency. Gastroenterology. 1995;109:282–4.
Davies SM, Szabo E, Wagner JE, Ramsay NK, Weisdorf DJ. Idiopathic hyperammonemia: a frequently lethal complication of bone marrow transplantation. Bone Marrow Transplant. 1996;17:1119–25.
Mitchell RB, Wagner JE, Karp JE, Watson AJ, Brusilow SW, Przepiorka D, et al. Syndrome of idiopathic hyperammonemia after high-dose chemotherapy: review of nine cases. Am J Med. 1988;85:662–7.
Matsuzaki H, Uchiba M, Yoshimura K, Yoshida M, Akahoshi Y, Okazaki K, et al. Hyperammonemia in multiple myeloma. Acta Haematol. 1990;84:130–4.
Kwan L, Wang C, Levitt L. Hyperammonemic encephalopathy in multiple myeloma. N Engl J Med. 2002;346:1674–5.
Takimoto Y, Imanaka F, Hayashi Y, Morioka S. A patient with ammonia-producing multiple myeloma showing hyperammonemic encephalopathy. Leukemia. 1996;10:918–9.
Ho AY, Mijovic A, Pagliuca A, Mufti GJ. Idiopathic hyperammonaemia syndrome following allogeneic peripheral blood progenitor cell transplantation (allo-PBPCT). Bone Marrow Transplant. 1997;20:1007–8.
Liaw CC, Liaw SJ, Wang CH, Chiu MC, Huang JS. Transient hyperammonemia related to chemotherapy with continuous infusion of high-dose 5-fluorouracil. Anti-Cancer Drugs. 1993;4:311–5.
Espinos J, Rifon J, Perez-Calvo J, et al. Idiopathic hyperammonemia following high-dose chemotherapy. Bone Marrow Transplant. 2006;37:899.
Lichtenstein GR, Yang YX, Nunes FA, Lewis JD, Tuchman M, Tino G, et al. Fatal hyperammonemia after orthotopic lung transplantation. Ann Intern Med. 2000;132:283–7.
del Rosario M, Werlin SL, Lauer SJ. Hyperammonemic encephalopathy after chemotherapy: survival after treatment with sodium benzoate and sodium phenylacetate. J Clin Gastroenterol. 1997;25:682–4.
Vaquero J, Blei AT. Mild hypothermia for acute liver failure: a review of mechanisms of action. J Clin Gastroenterol. 2005;39:S147–57.
Salvi S, Santorelli FM, Bertini E, Boldrini R, Meli C, Donati A, et al. Clinical and molecular findings in hyperornithinemia-hyperammonemia-homocitrullinuria syndrome. Neurology. 2001;57:911–4.
Lemay JF, Lambert MA, Mitchell GA, Vanasse M, Valle D, Arbour JF, et al. Hyperammonemia-hyperornithinemia-homocitrullinuria syndrome: neurologic, ophthalmologic, and neuropsychologic examination of six patients. J Pediatr. 1992;121:725–30.
Smith W, Kishnani PS, Lee B, Singh RH, Rhead WJ, Sniderman King L, et al. Urea cycle disorders: clinical presentation outside the newborn period. Crit Care Clin. 2005;21:S9–S17.
Blanco Vela CI, Bosques Padilla FJ. Determination of ammonia concentrations in cirrhosis patients-still confusing after all these years? Ann Hepatol. 2011;10(Suppl 2):S60–5.
Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, van Lente F, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188–93.
Haberle J. Clinical and biochemical aspects of primary and secondary hyperammonemic disorders. Arch Biochem Biophys. 2013;536(2):101–8.
Colombo JP, Peheim E, Kretschmer R, Dauwalder H, Sidiropoulos D. Plasma ammonia concentrations in newborns and children. Clin Chim Acta. 1984;138(3):283–91.
Häberle J, Boddaert N, Burlina A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7:32.
Alfadhel M, Mutairi FA, Makhseed N. Guidelines for acute management hyperammonemia in the Middle East region. Ther Clin Risk Manag. 2016;12:479–87.
Naylor EW, Chace DH. Automated tandem mass spectrometry for mass newborn screening for disorders in fatty acid, organic acid, and amino acid metabolism. J Child Neurol. 1999;14(Suppl 1):S4–8.
Wraith JE. Ornithine carbamoyltransferase deficiency. Arch Dis Child. 2001;84:84–8.
Steiner RD, Cederbaum SD. Laboratory evaluation of urea cycle disorders. J Pediatr. 2001;138:S21–9.
Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol. 2000;15(9):969–79.
Laish I, Ben AZ. Noncirrhotic hyperammonaemic encephalopathy. Liver Int. 2011;31(9):1259–70.
Upadhyay R, Bleck TP, Busl KM. Hyperammonemia: what urea-lly need to know: case report of severe noncirrhotic hyperammonemic encephalopathy and review of the literature. Case Rep Med. 2016;2016:8512721.
U-King-Im JM, Yu E, Bartlett E, Soobrah R, Kucharczyk W. Acute hyperammonemic encephalopathy in adults: imaging findings. AJNR Am J Neuroradiol. 2011;32(2):413–8.
Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35.
Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;2:CD003044.
Leise MD, Poterucha JJ, Kamath PS, Kim WR. Management of hepatic encephalopathy in the hospital. Mayo Clin Proc. 2014;89:241–53.
•• Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016; (5):CD003044. This was a meta-analysis for 38 randomized clinical trials that were selected based on GRADE criteria. Th authors compared non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Random-effects meta-analysis showed a beneficial effect of non-absorbable disaccharides on mortality, hepatic encephalopathy compared to placebo. In addition, non-absorbable disaccharides can help to reduce serious adverse events associated with the underlying liver disease including liver failure, hepatorenal syndrome, and variceal bleeding.
Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328(7447):1046.
Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. Lactulose vs polyethylene glycol 3350—electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–33.
Gupta T, Rathi S, Dhiman RK. Managing encephalopathy in the outpatient setting. Euroasian J Hepato-Gastroenterol. 2017;7(1):48–54.
Kimer N, Krag A, Gluud LL. Safety, efficacy, and patient acceptability of rifaximin for hepatic encephalopathy. Patient Prefer Adherence. 2014;8:331–8.
Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trial. Am J Dig Dis. 1978;23(5):398–406.
Last PM, Sherlock S. Systemic absorption of orally administered neomycin in liver disease. N Engl J Med. 1960;262:385–9.
Kim KH, Choi JW, Lee JY, Kim TD, Paek JH, Lee EJ, et al. Two cases of metronidazole-induced encephalopathy. Korean J Gastroenterol. 2005;45(3):195–200.
Uhl MD, Riely CA. Metronidazole in treating portosystemic encephalopathy. Ann Intern Med. 1996;124:455.
Tarao K, Ikeda T, Hayashi K, Sakurai A, Okada T, Ito T, et al. Successful use of vancomycin hydrochloride in the treatment of lactulose resistant chronic hepatic encephalopathy. Gut. 1990;31(6):702–6.
Lester W. Rifampin: a semisynthetic derivative of rifamycin—a prototype for the future. Annu Rev Microbiol. 1972;26:85–102.
Marchi E, Montecchi L, Venturini AP, et al. 4-Deoxypyrido[1′,2′:1,2]imidazo[5,4-c]rifamycin SV derivatives. A new series of semisynthetic rifamycins with high antibacterial activity and low gastroenteric absorption. J Med Chem. 1985;28(7):960–3.
Bucci L, Palmieri GC. Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Curr Med Res Opin. 1993;13(2):109–18.
Pedretti G, Calzetti C, Missale G, et al. Rifaximin versus neomycin on hyperammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Ital J Gastroenterol. 1991;23(4):175–8.
Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51–8.
Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–81.
Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–63.
Kimer N, Krag A, Møller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40(2):123–32.
Blandizzi C, Viscomi GC, Marzo A, Scarpignato C. Is generic rifaximin still a poorly absorbed antibiotic? A comparison of branded and generic formulations in healthy volunteers. Pharmacol Res. 2014;85:39–44.
Huang DB, DuPont HL. Rifaximin—a novel antimicrobial for enteric infections. J Inf Secur. 2005;50(2):97–106.
Descombe JJ, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res. 1994;14(2):51–6.
Ahire K, Sonawale A. Comparison of rifaximin plus lactulose with the lactulose alone for the treatment of hepatic encephalopathy. J Assoc Physicians India. 2017;65(8):42–6.
Williams R, James OF, Warnes TW. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12(2):203–8.
Paik YH, Lee KS, Han KH, Song KH, Kim MH, Moon BS, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46(3):399–407.
Zhu GQ, Shi KQ, Huang S, Wang LR, Lin YQ, Huang GQ, et al. Systematic review with network meta-analysis: the comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Aliment Pharmacol Ther. 2015;41(7):624–35.
Brusilow SW, Valle DL, Batshaw M. New pathways of nitrogen excretion in inborn errors of urea synthesis. Lancet. 1979;2(8140):452–4.
Barshop BA, Breuer J, Holm J, Leslie J, Nyhan WL. Excretion of hippuric acid during sodium benzoate therapy in patients with hyperglycinaemia or hyperammonaemia. J Inherit Metab Dis. 1989;12:72–9.
Webster LT, Siddiqui UA, Lucas SV, Strong JM, Mieyal JJ. Identification of separate Acyl-CoA:Glycine and Acyl-CoA:L-glutamine N-acyltransferase activities in mitochondrial fractions from liver of rhesus monkey and man. J Biol Chem. 1976;251:3352–8.
Walker V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes Metab. 2009;11:823–35.
• Kristiansen RG, Rose CF. Ytrebo LM Glycine and hyperammonemia: potential target for the treatment of hepatic encephalopathy. Metab Brain Dis. 2016;31:1269–73. The authors discussed the role of Ornithine Phenylacetate in reducing the arterial ammonia levels in patients with liver failure via modulating glutamine levels. This imposes a promising role for glycine in the pathogenesis of HE which merits further investigations.
Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, Hamosh A. Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med. 2007;356:2282–92.
• Pena-Quintana L, Llarena M, Reyes-Suarez D, Aldamiz-Echevarria L. Profile of sodium phenylbutyrate granules for the treatment of urea-cycle disorders: patient perspectives. Patient Prefer Adherence. 2017;11:1489–96. Authors discussed recently developed taste-masked formulation of sodium phenylbutyrate granules was designed to overcome the considerable issues that taste has on adherence to therapy. Analysis for the most recent studies showed improved compliance, efficacy, and safety with this new product.
Newmark HL, Young CW. Butyrate and phenylacetate as differentiating agents: practical problems and opportunities. J Cell Biochem Suppl. 1995;22:247–53.
Guffon N, Kibleur Y, Copalu W, Tissen C, Breitkreutz J. Developing a new formulation of sodium phenylbutyrate. Arch Dis Child. 2012;97(12):1081–5.
McGuire BM, Zupanets IA, Lowe ME, et al. Pharmacology and safety of glycerol phenylbutyrate in healthy adults and adults with cirrhosis. Hepatology. 2010;51(6):2077–85.
Weiss N, Tripon S, Lodey M, et al. Treating hepatic encephalopathy in cirrhotic patients admitted to ICU with sodium phenylbutyrate (PB): a preliminary study. Fundam Clin Pharmacol. 2018;32(2):209–15. This is a prospective matched control data that aimed to assess the benefits of sodium PB in cirrhotic patients admitted to ICU for overt HE. The authors assessed the following outcomes: ammonia levels decrease, neurological improvement, and survival. They have found that sodium PB could be effective in reducing ammonia levels as well as in improving neurological status and ICU survival.
Matoori S, Leroux JC. Recent advances in the treatment of hyperammonemia. Adv Drug Deliv Rev. 2015;90:55–68.
Rose C, Michalak A, Pannunzio P, Therrien G, Quack G, Kircheis G, et al. L-ornithine-L-aspartate in experimental portal-systemic encephalopathy: therapeutic efficacy and mechanism of action. Metab Brain Dis. 1998;13(2):147–57.
Alvares-da-Silva MR, de Araujo A, Vicenzi JR, da Silva GV, Oliveira FB, Schacher F, et al. Oral L-ornithine-L-aspartate in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:956–63.
Mittal VV, Sharma BC, Sharma P, Sarin SK. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2011;23(8):725–32.
Kircheis G, Nilius R, Held H, et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology. 1997;25(6):1351–60.
Stauch S, Kircheis G, Adler G, Beckh K, Ditschuneit H, Görtelmeyer R, et al. Oral L-ornithine-L-aspartate therapy of chronic hepatic encephalopathy: resultsof a placebo-controlled double-blind study. J J Hepatol. 1998;28(5):856–64.
Jiang Q, Jiang XH, Zheng MH, Chen YP. L-Ornithine-l-aspartate in the management of hepatic encephalopathy: a meta-analysis. J Gastroenterol Hepatol. 2009;24:9–14.
Daniotti M, la Marca G, Fiorini P, Filippi L. New developments in the treatment of hyperammonemia: emerging use of carglumic acid. Int J Gen Med. 2011;4:21–8.
Kasapkara CS, Kanğın M, Taş FF, et al. Unusual cause of hyperammonemia in two cases with short-term and long-term valproate therapy successfully treated by single dose carglumic acid. J Pediatr Neurosci. 2013;8(3):250–2.
Kose E, Kuyum P, Aksoy B, Häberle J, Arslan N, Ozturk Y. First report of carglumic acid in a patient with citrullinemia type 1 (argininosuccinate synthetase deficiency). J Clin Pharm Ther. 2018;43(1):124–8.
Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39(5):1441–9.
Effect of antibiotics, prebiotics and probiotics in treatment forhepatic encephalopathy. Med. Hypotheses 2005, 64(1):64–68.
McGee RG, Bakens A, Wiley K. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev. 2011;11:CD008716.
Dalal R, McGee RG, Riordan SM, et al. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716.
Dhiman RK, Rana B, Agrawal S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147(6):1327–37.e3.
Chapman TM, Plosker GL, Figgitt DP. VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases. Drugs. 2006;66(10):1371–87.
Takuma Y, Nouso K, Makino Y, Hayashi M, Takahashi H. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther. 2010;32(9):1080–90.
Marchesini G, Fabbri A, Bianchi G, Brizi M, Zoli M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology. 1996;23(5):1084–92.
Riggio O, Ariosto F, Merli M, Caschera M, Zullo A, Balducci G, et al. Short-term oral zinc supplementation does not improve chronic hepatic encephalopathy. Results of a double-blind crossover trial. Dig Dis Sci. 1991;36(9):1204–8.
Bresci G, Parisi G, Banti S. Management of hepatic encephalopathy with oral zinc supplementation: a long-term treatment. Eur J Med. 1993;2(7):414–6.
Reding P, Duchateau J, Bataille C. Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial. Lancet. 1984;2(8401):493–5.
Katayama K, Saito M, Kawaguchi K, et al. Effect of zinc on liver cirrhosis with hyperammonemia: a preliminary randomized, placebo-controlled double-blind trial. Nutrition. 2014;30(11–12):1409–14.
Malaguarnera M. Acetyl-L-carnitine in hepatic encephalopathy. Metab Brain Dis. 2013;28(2):193–9.
Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci. 2004;1033:30–41.
Gidal BE, Inglese CM, Meyer JF, Pitterle ME, Antonopolous J, Rust RS. Diet- and valproate-induced transient hyperammonemia: effect of L-carnitine. Pediatr Neurol. 1997;16(4):301–5.
Jiang Q, Jiang G, Shi KQ, Cai H, Wang YX, Zheng MH. Oral acetyl-L-carnitine treatment in hepatic encephalopathy: view of evidence-based medicine. Ann Hepatol. 2013;12(5):803–9.
Summar ML, Dobbelaere D, Brusilow S, Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr. 2008;97(10):1420–5.
Legras A, Labarthe F, Maillot F, Garrigue MA, Kouatchet A, Ogier de Baulny H. Late diagnosis of ornithine transcarbamylase defect in three related female patients: polymorphic presentations. Crit Care Med. 2002;30(1):241–4.
Wilnai Y, Blumenfeld YJ, Cusmano K, Hintz SR, Alcorn D, Benitz WE, et al. Prenatal treatment of ornithine transcarbamylase deficiency. Mol Genet Metab. 2018;123(3):297–300.
Jalan R, Wright G, Davies NA, Hodges SJ. L-Ornithine phenylacetate (OP): a novel treatment for hyperammonemia and hepatic encephalopathy. Med Hypotheses. 2007;69(5):1064–9.
Ventura-Cots M, Arranz JA, Simón-Talero M, Torrens M, Blanco A, Riudor E, et al. Safety of ornithine phenylacetate in cirrhotic decompensated patients: an open-label, dose-escalating, single-cohort study. J Clin Gastroenterol. 2013;47(10):881–7.
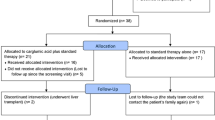
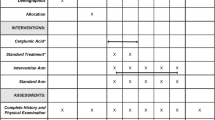
• Ventura-Cots M, Concepción M, Arranz JA, et al. Impact of ornithine phenylacetate (OCR-002) in lowering plasma ammonia after upper gastrointestinal bleeding in cirrhotic patients. Therap Adv Gastroenterol. 2016;9(6):823–35. This is a prospective study that aimed to study the effect of ornithine phenylacetate on plasma ammonia levels after upper GI bleed in patients with cirrhosis. They included 38 patients in this study and found no difference between the treatment arm as compared to the glucosaline arm in venous plasma ammonia level at 24hrs after treatment.
Montet J, Salmon L, Caroli-Bosc FX, et al. L-arginine infusion protects against hyperammonemia and hepatic encephalopathy in cirrhotic patients with variceal haemorrhage. Gastroenterology. 2000;118(4):A978.
Campollo O, Sprengers D, McIntyre N. The BCAA/AAA ratio of plasma amino acids in three different groups of cirrhotics. Rev Investig Clin. 1992;44(4):513–8.
Liu J, Lkhagva E, Chung HJ. The pharmabiotic approach to treat hyperammonemia. Nutrients 2018 ;10(2).
Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. World J Gastroenterol. 2013;19(43):7620–9.
Frazier TH, Wheeler BE, McClain CJ, Cave M. Liver disease. In: Mueller CM,ed. The A.S.P.E.N. adult nutrition support core curriculum. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition; 2012:454–471.
Freund H, Hoover HC Jr, Atamian S, et al. Infusion of the branched chain amino acids in postoperative patients. Anticatabolic properties. Ann Surg. 1979;190(1):18–23.
Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Phys. 1992;263(5 Pt 1):E928–34.
• Gluud LL, Dam G, Les I, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. Meta-analysis for 16 studies that included 827 patients with HE who were treated with oral and intravenous BCAA. No difference was shown in mortality in a random-effects meta-analysis. However, a beneficial effect of BCAA was noticed on hepatic encephalopathy resolution but with increased risk of nausea and vomiting.
Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, et al. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. The Italian Multicenter Study Group. J Hepatol. 1990;11(1):92–101.
Jalan R, Kapoor D. Enhanced renal ammonia excretion following volume expansion in patients with well compensated cirrhosis of the liver. Gut. 2003;52(7):1041–5.
Jalan R, Kapoor D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci (Lond). 2004;106(5):467–74.
van Straten G, de Sain-van der Velden MGM, van Geijlswijk IM, Favier RP, Mesu SJ, Holwerda-Loof NE, et al. Saline is as effective as nitrogen scavengers for treatment of hyperammonemia. Sci Rep. 2017;7(1):13112.
Bajaj JS, Sheikh MY, Chojkier M, et al. AST-120(spherical carbon adsorbent) in covert hepatic encephalopathy: results of the ASTUTE trial. J Hepatol. 2013;58:S84.
Ampuero J, Ranchal I, Nuñez D, et al. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PLoS One. 2012;7(11):e49279.
Mitzner SR. Extracorporeal liver support-albumin dialysis with the Molecular Adsorbent Recirculating System (MARS). Ann Hepatol. 2011;10(Suppl 1):S21–8.
Bañares R, Catalina MV, Vaquero J. Molecular adsorbent recirculating system and bioartificial devices for liver failure. Clin Liver Dis. 2014;18(4):945–56.
Lee EK, Hu C, Bhargava R, Rozengurt N, Stout D, Grody WW, et al. Long-term survival of the juvenile lethal arginase-deficient mouse with AAV gene therapy. Mol Ther. 2012;20(10):1844–51.
Sin YY, Price PR, Ballantyne LL, et al. Proof-of-concept gene editing for the murine model of inducible arginase-1 deficiency. Sci Rep. 2017;7(1):2585.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacology of Acute Care
Rights and permissions
About this article
Cite this article
Alshaya, A., Fanikos, J. & DeMaio, E. Intravenous and Oral Hyperammonemia Management. Curr Emerg Hosp Med Rep 6, 182–193 (2018). https://doi.org/10.1007/s40138-018-0174-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-018-0174-5