Abstract
Purpose of Review
We will review the pharmacodynamics and clinical outcomes of morphine therapy for pulmonary oedema.
Recent Findings
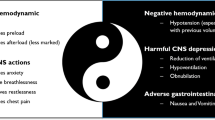
Both animal and human studies demonstrate that morphine has vasodilatory properties. The effect on pulmonary hemodynamics seems to be neutral and possibly adverse on ventilation. Morphine, along with furosemide and nitrates, is routinely used to treat cardiogenic pulmonary oedema. Clinical data on the safety and efficacy of morphine for cardiogenic pulmonary oedema are scarce; however, morphine use has been correlated with increased rates of ICU admission and mechanical ventilation. European and American heart failure guidelines do not recommend routine use of morphine for cardiogenic pulmonary oedema.
Summary
Morphine is of questionable benefit and may be harmful in treatment of acute pulmonary oedema. Clinical guidelines do not encourage routine use of morphine for pulmonary oedema; other medications for anxiolysis and vasodilation may be preferable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiogenic pulmonary oedema (CPE) is the second most common cause of dyspnoea presenting to the emergency department [1, 2]. Therapy usually targets correction of gas exchange via invasive or non-invasive ventilation, diuresis, and altering pulmonary vascular hemodynamics to decrease capillary leakage [3, 4]. Morphine has been described to be beneficial in these cases since the 1950s; however, concerns have been raised regarding its efficacy and safety [5, 6]. We will review the pathophysiology of pulmonary oedema, the rationale of using morphine for CPE, and relevant pharmacodynamic and clinical outcome data.
Pathophysiology of Pulmonary Oedema
CPE can be defined as increased fluid content of the lung interstitium secondary to increased left atrial pressure. Manifestations include increased intra-alveolar fluid, decreased pulmonary compliance, and ventilation perfusion mismatch [7]. In normal physiology, a trivial amount of fluid transfers from the pulmonary vasculature into the lung interstitium that is cleared by the pulmonary lymphatics at a rate of roughly 20 ml/hr [8]. When intracapillary hydrostatic pressure is high, extravascular fluid shifts are increased [9]. Alveolar fluid accumulation tends to occur when capillary pressure exceeds 20 mmHg [10, 11]. Elevation in capillary pressure is often due to increased left atrial pressure which is often due to left ventricular failure. This can be acute or chronic and due to heart failure with preserved or reduced ejection fraction. When capillary pressure and fluid extravasation overwhelm the lymphatic system capacitance, overt CPE typically occurs. Recovery and resolution can be complicated if heart failure hinders pulmonary lymphatic outflow as it drains in the venous system, i.e., increases lymphatic pressure afterload [12].
Morphine Effect on Vasculature and Hemodynamics
The vascular response to morphine was described by Vasko et al. who induced cardiogenic pulmonary oedema in 18 dogs before administering 1 mg/kg of morphine [13]. Canine subjects were mechanically ventilated and underwent invasive left and right cardiac pressure monitoring. Although there was mild to moderate decrease in pulmonary vascular resistance, the predominant hemodynamic response was preload reduction via increasing the capacitance of peripheral venous circulation. This was further investigated by Greenberg et al. who compared morphine and furosemide for their effect on vascular smooth muscles in vivo [14]. They used isolated rings of canine pulmonary, mesenteric, splenic, and anterior tibial arteries and veins. Furosemide was found to have its most relaxing effect on pulmonary veins via an endothelium independent mechanism. Morphine, on the other hand, had a relaxing effect on pulmonary arterial and venous tissues, mainly by increasing prostanoid release from the endothelium.
Zelis et al. studied the morphine response in the upper extremity vasculature of 69 human subjects [15]. Morphine was observed to induce rapid vasoconstriction lasting 1–2 min followed by a 35% reduction in the venous pressure and a 25% reduction in vascular resistance at 10 min. Arterial blood pressure remained constant, resulting in a 26% increase in blood flow. The physiologic vasoconstrictor response to deep breathing, mental arithmetic, cold, post-Valsalva overshoot, and 45° head-up position was intact. The team attempted to describe the mechanism of morphine’s action by infusing 200 μg/min in the brachial artery and adding promethazine (an antihistamine), propranolol (a beta-adrenergic blocker), and atropine (a cholinergic blocker). No effect was observed on morphine vasodilatory action; however, phentolamine (an alpha-adrenergic agonist) abolished the effect. This suggests that morphine causes vasodilation by reducing central sympathetic efferent discharge. Vismara et al. used a similar technique to compare the vascular response to morphine in 13 subjects with pulmonary oedema and normal subjects. Morphine sulfate was infused at 0.1 mg/kg and a similar venodilatory response was observed; however, there was no significant difference between those with pulmonary oedema and controls [16]. Morphine was also shown to decrease splanchnic vascular resistance resulting in a 19% increase in splanchnic blood flow without a change in systemic or right atrial pressure when infused at a dose of 0.2 mg/kg to a maximum of 15 mg in 13 patients [17]. A later investigation by Grossmann et al. demonstrated that infusing morphine with naloxone did not alter its vasodilatory effect on hand veins of healthy volunteers [18]. Nonetheless, co-infusion with a combination of diphenhydramine (an H1 receptor blocker) and famotidine (an H2 receptor blocker) blunted the vascular response, indicating a histamine-mediated effect, contrary to the results of Zelis et al. Fentanyl did not have a significant effect on peripheral vasculature.
Studies on morphine’s effect on cardiac function have also shown discrepant results. Lappas et al. studied eight patients with myocardial ischemia requiring revascularization and who had normal baseline systolic function [19]. Right and left cardiac filling pressures increased with a morphine dose of 1.5 mg/kg or more. However, stroke volume, cardiac output, and systemic arterial pressure decreased after a dose of 0.5 mg/kg. Systemic vascular resistance was unchanged indicating that blood pressure decrement was secondary to decreased cardiac output rather than vasodilation. Correspondingly, other research has shown that inhibition of CNS opioid receptors may increase blood pressure and cardiac output [20]. More clinically relevant data were presented by Lee and colleagues who administered morphine 15 mg to ten patients with acute transmural myocardial infarction, four were Killip class I, three were Killip class II, and three were Killip class III [21••]. Invasive and echocardiographic hemodynamic assessment showed no change in pulmonary capillary wedge pressure, ejection fraction, left ventricular dimensions, or right and left filling pressures. There was, however, a slight increase in pulmonary vascular resistance at 45 min post injection from 132.4 ± 13.8 to 183.1 ± 3.2. Morphine was studied in a cohort of ten patients with acute myocardial infarction complicated by systolic dysfunction; each received a dose of 0.2 mg/kg morphine. Subjects had mild negative effect on HR, BP, and SV, and no effect on LV filling pressure [22]. Due to inconsistent evidence, the act of morphine to relieve cardiac dyspnoea cannot be adequately explained by pulmonary vascular preload reduction. Anxiolysis may be an important contributor, however, literature to support this hypothesis is scant [6, 23].
Effect on Gas Exchange Function
Several studies have shown that morphine causes decreased respiratory rate and tidal volume and blunts hypoxic and hypercapnic ventilatory responses [24,25,26,27,28]. This is thought to be mediated by its agonistic action on μ-receptors located in the central nervous system [29, 30]. Recently, Zhuang et al. verified a heavy expression of μ-receptors in the caudomedial nucleus tractus solitarius in rats [31]. This nucleus has chemosensitive neurons activated by hypercapnia and receives input from bronchopulmonary nerve fibres and carotid chemoreceptors involved in respiratory regulation. The study showed that microinjection of a μ-agonist into the nucleus significantly decreased baseline minute ventilation by 18% (P < 0.01). Hypoxic ventilatory response was profoundly attenuated by 70% due to reduction in both respiratory frequency (47%) and minute ventilation (77%). Hypercapnic ventilator response was attenuated by 21%.
By causing hypoventilation, morphine can lead to respiratory acidosis. Patients with severe acidotic acute CPE who received opioids have had slower improvement in pH but there was no increase in respiratory distress or 7-day mortality [32]. On the other hand, supplemental oxygen given to pulmonary oedema patients may exacerbate opioid-induced respiratory depression. Niesters et al. demonstrated that healthy volunteers receiving hyperoxic air supplements have greater opioid-induced respiratory depression compared to volunteers receiving normoxic supplements [33]. These findings are clinically relevant as patients with signs of respiratory distress are routinely given oxygen supplementation, even in the absence of hypoxemia [34].
Clinical Outcomes of Using Morphine in Cardiogenic Pulmonary Oedema
Morphine use in CPE has been encouraged based on clinical observations of relief of respiratory distress in the absence of data that demonstrates efficacy [5, 35, 36]. The mnemonic “MONA” encouraged morphine, oxygen, nitrates, and aspirin for treatment of acute myocardial infarction, although the origins of this memory device are unknown [37]. Table 1 summarizes the available clinical evidence of using morphine for CPE. One major hurdle to studying the efficacy of prehospital CPE-specific therapies is a 23–40% rate of prehospital misdiagnosis of acute obstructive lung disease, infection, or other types of pulmonary oedema as CPE [39, 41, 45]. A prospective study of 57 patients presenting with acute respiratory failure evaluated prehospital administration of different combinations of morphine, furosemide, and nitrates and showed no benefit of morphine and indicated a signal for worsening ventilation [41]. Another prospective evaluation of 84 patients receiving morphine for suspected CPE by paramedics illustrated the potential for adverse effects. Respiratory depression was observed in one patient who received morphine by paramedics and was ultimately diagnosed with aspiration pneumonia by the physician’s assessment upon arrival in the ED [39]. A study of prehospital morphine safety examined the prehospital treatment regimens of 319 patients with ADHF; 6% received prehospital morphine with no independent association to change in vital signs or clinical outcomes [45].
Data on in-hospital morphine usage are discouraging. Use of morphine in the emergency department for pulmonary oedema is correlated with higher likelihood of ICU admission (odds ratio 3.08, P = 0.002) and mechanical ventilation (odds ratio 5.04, P = 0.001) [44]. While data do not demonstrate a causal relationship, this observation may be relevant to rural areas where advanced critical care resources may be limited [46]. A study of morphine usage in 4102 patients admitted to the hospital with acute heart failure decompensation observed that the 9.3% who received morphine were more likely to have acute coronary syndrome, acute chronic heart failure, diabetes mellitus, and hyperlipidaemia [42•]. Unadjusted in-hospital mortality was higher (odds ratio 2.0, 1.1– 3.5, P = 0.02). However, multivariate analysis showed no association between morphine and in-hospital mortality. A landmark retrospective analysis of 147,362 patients from the Acute Decompensated Heart Failure National Registry (ADHERE) has shown that 14.1% of CHF patients received morphine during their hospitalization [43••]. These patients had a higher prevalence of pulmonary oedema and positive troponin and were more likely to require mechanical ventilation (15.4 vs 2.8%, P < 0.001), longer median hospitalizations (5.6 vs 4.2 days, P < 0.001), and ICU admission (38.7 vs 14.4%, P < 0.001), as well as greater mortality (odds ratio 4.84, P < 0.001). Despite adjusting the data for demographics, laboratory values, and systolic function with similar results, there is still a significant chance of selection bias. The database does not include timing of morphine administration and cannot determine if morphine was given with end-of-life palliative intention [47, 48].
Based on the available data, the prevailing opinion is that morphine for CPE should be used secondary to other safer medications for anxiolysis and vasodilation [47,48,49,50,51]. The 2016 European Society of Cardiology does not recommend routine use of morphine for acute heart failure and only recommends cautious use of morphine in severe dyspnoea with pulmonary oedema [52]. Similarly, the Heart Failure Society of America in their 2010 guidelines advice caution if morphine is used in acute heart failure [53].
Conclusion
Morphine is often used for CPE to relieve dyspnoea and is presumed to provide a pulmonary vasodilatory effect. The available evidence fails to show any clear outcome benefit and suggests a potential for harm. Current guidelines recommend cautious use of morphine for CPE. Further study of morphine for CPE in randomized trials may be warranted.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kelly AM, et al. Epidemiology, prehospital care and outcomes of patients arriving by ambulance with dyspnoea: an observational study. Scand J Trauma Resusc Emerg Med. 2016;24(1):113.
Kelly AM, et al. An observational study of dyspnoea in emergency departments: the Asia, Australia, and New Zealand Dyspnoea in Emergency Departments study (AANZDEM). Acad Emerg Med. 2016.
Johnson MR. Acute pulmonary edema. Curr Treat Options Cardiovasc Med. 1999;1(3):269–76.
Allison RC. Initial treatment of pulmonary edema: a physiological approach. Am J Med Sci. 1991;302(6):385–91.
Sosnowski MA. Review article: lack of effect of opiates in the treatment of acute cardiogenic pulmonary oedema. Emerg Med Australas. 2008;20(5):384–90.
Ellingsrud C, Agewall S. Morphine in the treatment of acute pulmonary oedema—why? Int J Cardiol. 2016;202:870–3.
Fishman AP. Pulmonary edema. The Water-Exchanging Function of the Lung. 1972;46(2):390–408.
Staub NC. Pulmonary edema: physiologic approaches to management. Chest. 1978;74(5):559–64.
Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19(4):312–26.
Taylor AE, et al. The pulmonary interstitium in capillary exchange. Ann N Y Acad Sci. 1982;384:146–65.
Laine GA, et al. Effect of systemic venous pressure elevation on lymph flow and lung edema formation. J Appl Physiol (1985). 1986;61(5):1634–8.
Laine GA, et al. Effect of systemic venous pressure elevation on lymph flow and lung edema formation. J Appl Physiol. 1986;61(5):1634–8.
Vasko JS, et al. Mechanisms of action of morphine in the treatment of experimental pulmonary edema. Am J Cardiol. 1966;18(6):876–83.
Greenberg S, et al. Selective pulmonary and venous smooth muscle relaxation by furosemide: a comparison with morphine. J Pharmacol Exp Ther. 1994;270(3):1077–85.
Zelis R, et al. The cardiovascular effects of morphine the peripheral capacitance and resistance vessels in human subjects. J Clin Invest. 54(6):1247–58.
Vismara LA, Leaman DM, Zelis R. The effects of morphine on venous tone in patients with acute pulmonary edema. Circulation. 1976;54(2):335–7.
Leaman DM, et al. Effect of morphine on splanchnic blood flow. Br Heart J. 1978;40(5):569–71.
Grossmann M, et al. Morphine-induced venodilation in humans. Clin Pharmacol Ther. 1996;60(5):554–60.
Lappas MD, Demetrios G, et al. Filling pressures of the heart and pulmonary circulation of the patient with coronary-artery disease after large intravenous doses of morphine. Anesthesiology. 1975;42(2):153–9.
Pugsley MK. The diverse molecular mechanisms responsible for the actions of opioids on the cardiovascular system. Pharmacol Ther. 2002;93(1):51–75.
•• Lee G, et al. Comparative effects of morphine, meperidine and pentazocine on cardiocirculatory dynamics in patients with acute myocardial infarction. Am J Med. 1976;60(7):949–55. A prospective and invasive examination of the hemodynamic effects of opioids in patients with acute coronary syndrome with and without clinical pulmonary edema that showed no major changes in left or right intracardiac and central pressures
Timmis AD, et al. Haemodynamic effects of intravenous morphine in patients with acute myocardial infarction complicated by severe left ventricular failure. Br Med J. 1980;280(6219):980–2.
Mattu A, Martinez JP, Kelly BS. Modern management of cardiogenic pulmonary edema. Emerg Med Clin North Am. 2005;23(4):1105–25.
Weil JV, et al. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med. 1975;292(21):1103–6.
Modalen ÅÖ, et al. A novel molecule with peripheral opioid properties: the effects on hypercarbic and hypoxic ventilation at steady-state compared with morphine and placebo. Anesth Analg. 2006;102(1):104–9.
Romberg R, et al. Pharmacodynamic effect of morphine-6-glucuronide versus morphine on hypoxic and hypercapnic breathing in healthy volunteers. Anesthesiology. 2003;99(4):788–98.
Shook JE, Watkins WD, Camporesi EM. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am Rev Respir Dis. 1990;142(4):895–909.
Hoel BL, Bay G, Refsum HE. The effects of morphine on the arterial and mixed venous blood gas state and on the hemodynamics in patients with clinical pulmonary congestion. Acta Med Scand. 1971;190(6):549–54.
Bailey PL, et al. Effects of intrathecal morphine on the ventilatory response to hypoxia. N Engl J Med. 2000;343(17):1228–34.
Takeda S, et al. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology. 2001;95(3):740–9.
Zhuang J, et al. Mu-opioid receptors in the caudomedial NTS are critical for respiratory responses to stimulation of bronchopulmonary C-fibers and carotid body in conscious rats. Respir Physiol Neurobiol. 2017;235:71–8.
Gray A, et al. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM. 2010;103(8):573–81.
Niesters M, et al. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth. 2013;110(5):837–41.
Branson RD, Johannigman JA. Pre-hospital oxygen therapy. Respir Care. 2013;58(1):86–97.
Klinefelter HF. Morphine for pulmonary edema. JAMA. 1974;229(6):638.
Adams Jr FB. Use of morphine sulfate for acute pulmonary edema. Am Fam Physician. 1997;55(5):1561.
Kline KP, Conti CR, Winchester DE. Historical perspective and contemporary management of acute coronary syndromes: from MONA to THROMBINS2. Postgrad Med. 2015;127(8):855–62.
Beltrame JF, et al. Nitrate therapy is an alternative to furosemide/morphine therapy in the management of acute cardiogenic pulmonary edema. J Card Fail. 1998;4(4):271–9.
Bruns BM, et al. Safety of pre-hospital therapy with morphine sulfate. Am J Emerg Med. 1992;10(1):53–7.
Fiutowski M, et al. Clinical presentation and pharmacological therapy in patients with cardiogenic pulmonary oedema. Kardiol Pol. 2004;61(12):561–9. discussion 570
Hoffman JR, Reynolds S. Comparison of nitroglycerin, morphine and furosemide in treatment of presumed pre-hospital pulmonary edema. Chest. 1987;92(4):586–93.
• Iakobishvili Z, et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care. 2011;13(2):76–80. Large retrospective regression analysis that showed an odds ratio of 2.0 (confidence interval 1.1–3.5) to increased mortality when morphine is used for acute heart failure. They also demonstrated that this cohort had higher percentage of acute myocardial ischemia and traditional risk factors of ischemic heart disease, so that with adjustment for propensity score, the analysis did not reach significance
•• Peacock WF, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25(4):205–9. The largest and most recent investigation linking morphine usage for CPE to mechanical ventilation (15.4 vs 2.8%), median hospitalisation (5.6 vs 4.2 days), ICU admissions, and mortality (13.0 vs 2.4%). All P values were <0.001. This reference was cited by the 2016 European Society of Cardiology heart failure guidelines
Sacchetti A, et al. Effect of ED management on ICU use in acute pulmonary edema. Am J Emerg Med. 1999;17(6):571–4.
Sporer KA, et al. Do medications affect vital signs in the prehospital treatment of acute decompensated heart failure? Prehosp Emerg Care. 2006;10(1):41–5.
Bosomworth J. Rural treatment of acute cardiogenic pulmonary edema: applying the evidence to achieve success with failure. Can J Rural Med. 2008;13(3):121–8.
Cattermole GN, Graham CA. Opiates should be avoided in acute decompensated heart failure. Emerg Med J. 2009;26(3):230–1.
Graham CA, Cattermole GN. Morphine should be abandoned as a treatment for acute cardiogenic pulmonary oedema. Emerg Med Australas. 2009;21(2):160.
Graham CA. Pharmacological therapy of acute cardiogenic pulmonary oedema in the emergency department. Emergency Medicine. 2004;16(1):47–54.
Chambers JA, Baggoley CJ. Pulmonary oedema—prehospital treatment. Caution with morphine dosage. Med J Aust. 1992;157(5):326–8.
Mosesso Jr VN, et al. Prehospital therapy for acute congestive heart failure: state of the art. Prehosp Emerg Care. 2003;7(1):13–23.
Ponikowski P, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC, 2016. 37(27) p 2129–2200.
Heart Failure Society of, A., HFSA. Comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Heart Failure
Rights and permissions
About this article
Cite this article
Al-Ani, M., Ismael, M. & Winchester, D.E. Morphine in Acute Pulmonary Oedema Treatment. Curr Emerg Hosp Med Rep 5, 88–93 (2017). https://doi.org/10.1007/s40138-017-0131-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-017-0131-8