Abstract
Background
Erector spinae plane block, a novel ultrasound-guided fascial plane block, has become popular for perioperative pain management. This randomized controlled trial tested the hypothesis that preoperative bilateral erector spinae plane block improves the quality of recovery in patients undergoing posterior lumbar interbody fusion.
Methods
Eighty-four patients scheduled for elective posterior lumbar interbody fusion were enrolled. Patients were randomly administered either ultrasound-guided bilateral erector spinae plane blocks using 20 ml of 0.375% ropivacaine on each side (ESPB group, n = 42) or no block (control group, n = 42) after anesthesia induction. The primary outcome was the quality of recovery 24 h postoperatively, assessed using the 15-item quality of recovery questionnaire.
Results
The global postoperative 24-h quality of recovery-15 score was 117 [114–121] in the erector spinae plane block group and 108 [105–111] in the control group, with a median difference of 9 (95% confidence interval 7–12, P < 0.001). Compared with the control group, preoperative bilateral erector spinae plane blocks reduced the area under the curve of the numeric rating scale pain scores over 48 h, prolonged the time to first rescue analgesia, lessened postoperative 24 h morphine consumption, decreased the occurrence of postoperative nausea and vomiting, and improved patient satisfaction with postoperative analgesia. There were no block-related adverse events.
Conclusion
We found that preoperative bilateral erector spinae plane blocks provided superior early quality of recovery, postoperative analgesia, and patient satisfaction scores in patients undergoing posterior lumbar interbody fusion.
Trial Registration
Chinese Clinical Trial Registry, ChiCTR1900027186, 4/11/2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Postoperative pain management remains a challenge for patients undergoing extensive spine surgery. |
Erector spinae plane block (ESPB) is an attractive opioid-sparing strategy for pain control after surgical procedures. |
We hypothesized that preoperative bilateral ultrasound-guided ESPB, compared with general anesthesia alone, would improve early recovery after posterior lumbar interbody fusion under general anesthesia. |
What was learned from the study? |
Preoperative bilateral ESPB enhances the quality of recovery and postoperative analgesia in patients undergoing posterior lumbar interbody fusion. |
It is reasonable to incorporate ESPB into a multimodal analgesic regimen in patients after extensive spine surgery. |
Introduction
As the population ages, lumbar degenerative disease is a common and debilitating ailment, causing pain and disability in patients [1]. Lumbar fusion surgery for degenerative conditions is steadily increasing over time, yet there is no consensus regarding the optimal postoperative analgesic regimen [2]. Postoperative acute pain is almost ubiquitous in some settings, especially in patients undergoing extensive spine surgery. Incompletely controlled postoperative pain may lead to delayed mobilization, postoperative pulmonary complications, prolonged hospital stays, and chronic pain syndromes [3]. The management of acute postoperative pain is an outstanding healthcare issue. Opioid-based intravenous patient-controlled analgesia is a frequent choice for pain control after lumbar spine surgery. However, opioids are associated with known side effects, including respiratory depression, nausea and vomiting, urinary retention, pruritus, constipation, and ileus [4]. To minimize surgical stress, reduce pain-related complications, and speed recovery, enhanced recovery after surgery pathways vigorously promote a multimodal analgesia regimen tailored to the patient [5]. Among the paths, regional anesthesia is a cornerstone strategy to provide excellent opioid-sparing analgesia.
Erector spinae plane block (ESPB), an increasingly popular technique for regional anesthesia and analgesia, is an ultrasound-guided interfascial block injecting local anesthetic into the plane below the erector spinae muscle [6]. ESPB is an attractive opioid-sparing strategy for pain control after surgical procedures [7]. Several recent studies have shown that ESPB is associated with improved postoperative analgesia outcomes in many surgeries [8], including lumbar spine surgery [9, 10]. The current evidence gap is whether the use of ESPB affects the patient-centered quality of recovery (QoR) following lumbar spinal surgery.
Thus, in this single-center, prospective randomized, parallel-group trial, we tested the primary hypothesis that preoperative bilateral ultrasound-guided ESPB, compared with general anesthesia alone, would improve early recovery (assessed using the 15-item QoR questionnaire) after posterior lumbar interbody fusion under general anesthesia by reducing acute postsurgical pain and opioid consumption.
Methods
Design and Patients
Ethical approval of this randomized controlled trial (K2019-09-026) was authorized by the Ethics Committee of Fujian Provincial Hospital, Fuzhou, China (Chairperson Prof. Lian Fayang) on 26 September 2019. We registered the study protocol at the Chinese Clinical Trial Registry (http://www.chictr.org.cn, identifier number: ChiCTR1900027186, 4/11/2019). The trial protocol is available in the Supplementary Information. This study was conducted at Fujian Provincial Hospital between November 2019 and March 2021, following the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting parallel-group randomized trials [11]. This trial was performed following the Declaration of Helsinki and Good Clinical Practice. Eligible participants were adults ≥ 18 years of age undergoing elective one- or two-level posterior lumbar interbody fusion, with the American Society of Anesthesiologists’ physical status of I–III. The patients underwent posterior decompression and interbody fusion using a minimally invasive surgical technique. Posterior decompression was achieved by partial laminectomy and unilateral or bilateral facetectomy. Interbody fusion was conducted using an interbody titanium cage filled with a local bone graft. All procedures were performed by the same surgeon. The exclusion criteria were as follows: (1) contraindications to regional blocks, such as coagulopathy and infection at the block site; (2) a history of opioid abuse; (3) a history of allergy to any trial drugs, such as local anesthetics and nonsteroidal anti-inflammatory drugs; (4) inability to communicate in Mandarin Chinese; (5) any other conditions that precluded study inclusion.
After providing written informed consent, patients were randomly allocated to receive bilateral ESPB using 20 ml of 0.375% ropivacaine on each side (ESPB group) or no block (control group) after anesthetic induction. Patients were assigned 1:1 in parallel groups using a computer-generated nonblocked randomization table created by an independent research assistant who was not involved in patient care or data collection. The group allocation codes were concealed in consecutively numbered opaque envelopes opened only on the morning of the surgery day by the nursing staff not involved in the study. ESPB was performed by a single anesthesiologist familiar with ESPB who was not involved in intraoperative anesthesia management or data collection. A second anesthesiologist was responsible for intraoperative anesthesia management and was not involved in data collection. After the time-out, the surgical team left the theatre for blinding purposes. The patients, surgeons, and investigators involved in data collection, analysis, and interpretation were unaware of the group allocation.
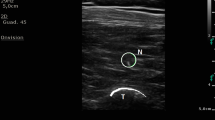
Ultrasound-Guided Erector Spinae Plane Block
Patients were gently placed in the prone position after induction of anesthesia, and bilateral ultrasound-guided ESPB was performed. Briefly, at the third lumbar vertebra level, a 2–5-MHz ultrasonic transducer (C60x, FUJIFILM SonoSite Inc., Bothell, WA, USA) was placed in a transverse orientation to identify the tip of the L3 transverse process. We then advanced a 21 gauge × 100 mm monopolar nerve blockade needle (USG-Type CCR, Hakko Co. Ltd, Chikuma-Shi, Nagano, Japan) into the deep plane of the erector spinae muscle using the in-plane technique. After a negative aspiration test for blood, 20 ml 0.375% ropivacaine was injected into the target interfascial plane under ultrasound guidance. Then, the procedure was duplicated on the contralateral side.
Anesthetic Procedure
The general anesthesia procedure and postoperative analgesic management schedule were standardized for all patients. Upon arrival in the operating room, the patients received monitoring, including electrocardiography, pulse oximetry, invasive blood pressure measurement, and bispectral index. We induced general anesthesia with sufentanil 0.5 μg kg−1, propofol 2.0 mg kg−1, and cisatracurium 0.15 mg kg−1. Following endotracheal intubation, dexamethasone 10 mg and tropisetron 5 mg were administered for antiemetic prophylaxis. Patients were mechanically ventilated using the pressure-controlled ventilation-volume guaranteed model to maintain an end-tidal carbon dioxide partial pressure between 35 and 45 mmHg. Anesthesia was maintained with inhaled sevoflurane (minimal alveolar concentration 0.8) and intravenous remifentanil infusion, targeting bispectral index values of 40–60 and hemodynamic parameters (heart rate and blood pressure) within 20% of the baseline value. Muscle relaxation was maintained by administering cisatracurium 5 mg at the discretion of the attending anesthesiologist. At the end of the surgery, the surgeon infiltrated the wounds using 10 ml of 0.25% ropivacaine. The neuromuscular blockade was antagonized using incremental doses of atropine and neostigmine if needed. Intravenous parecoxib 40 mg was administered before induction of anesthesia, followed by two times a day during the first 72 h after surgery. Additionally, postoperative analgesia was provided using morphine patient-controlled intravenous analgesia (PCIA) without a background infusion. Patients were instructed to utilize the PCIA bolus dose of morphine 2 mg, with a 10-min lockout interval (up to 10 mg per hour), if their numeric rating scale (NRS) pain score exceeded three at rest. When patients experienced postoperative nausea and vomiting (PONV), droperidol 0.625 mg and tropisetron 5 mg were intravenously administered as rescue antiemetics.
Outcome Assessment
The primary endpoint was QoR, assessed at 24 h postoperatively using the QoR-15 questionnaire [12]. Time zero was defined as the time when the surgical operation was completed. Questions included in this questionnaire incorporate physiologic values, functional recovery, and patient-reported outcomes. Each question is answered on an 11-point NRS score (0–10). The maximum global QoR-15 score is 150 (indicating the highest recovery quality). Secondary endpoints included the QoR-15 score 48 h postoperatively, the area under the curve (AUC) of NRS pain scores at rest and during mobilization over 48 h, the time to first rescue analgesia, intraoperative remifentanil consumption, postoperative 48 h morphine consumption, length of postanesthesia care unit (PACU) stay, patient satisfaction with postoperative analgesia, opioid-related side effects, and ESPB-related adverse events. Postoperative pain was assessed using an NRS score (range 0–10, 0 equals no pain and 10 equals the worst pain experienced) at 0.5, 1, 2, 4, 8, 24, and 48 h postoperatively. We defined the time to first rescue analgesia as the interval between emergence and the first PCIA dose of opioids. We determined the length of PACU stay as the interval from arrival to the PACU until the modified Aldrete scores were ≥ 9. Patient satisfaction with postoperative analgesia was evaluated at 48 h postoperatively using a verbal NRS score of 0–10 (0: completely unsatisfied; 10: the most satisfied). We explained all the above scoring criteria and confirmed that the patients understood preoperatively. A single specially trained investigator blinded to group assignment recorded all the above outcomes. Opioid-related side effects, including PONV, pruritus, and dizziness, were also questioned at the time of the pain evaluations by the blinded data collector during postoperative 48 h. Any ESPB-related adverse events, such as bleeding, infection, or local anesthetic systemic toxicity, were identified by the anesthesiologist responsible for ESPB during the study period and supplemented by the electronic medical record database review.
Statistical Analysis
The required sample size was calculated using the global QoR-15 score at 24 h postoperatively as the primary outcome variable. A clinically significant difference of 8 points in the global QoR-15 score between groups has been established [13]. Based on our pilot study, the global QoR-15 score 24 h postoperatively in the control group was 111 ± 10.6. We hypothesized that the ESPB group would have an 8-point improvement in the global QoR-15 score compared with the control group. Thus, 38 patients per group would provide a power of 80% with a type I error of 0.05. To compensate for the loss to follow-up, we ultimately recruited 84 patients for this study.
IBM SPSS Statistics for Windows software (version 25.0, IBM Corp., Armonk, NY, USA) was used for statistical analysis. The normality of the continuous data was evaluated using the Shapiro-Wilk test. Continuous data are reported as medians with interquartile ranges (IQRs) or means with standard deviations (SDs) where applicable; categorical data are reported as numbers (percentages, %). Groups were compared on continuous data using the Mann-Whiney U test with location shifts between groups (calculated using the Hodges-Lehmann estimate) and presented as 95% confidence intervals (CI). When appropriate, groups were compared on categorical data using Pearson chi-square or Fisher’s exact probability test. The AUCs of NRS pain scores over time were calculated using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). In addition, postoperative NRS pain scores were compared using a two-way repeated-measures analysis of variance. Bonferroni correction was applied for comparing groups at each time point, with P values adjusted by multiplying the nominal P value by the number of tests. All P values were two-tailed, and the criterion for rejection of the null hypothesis was P < 0.05.
Results
A total of 84 patients were enrolled between November 2019 and March 2021. One patient in the control group failed to complete the Q-15 questionnaire because of postoperative delirium. Consequently, we analyzed the results for 83 recruited patients (Fig. 1). Demographics, surgical characteristics, and preoperative QoR-15 scores were similar between groups (Table 1).
At 24 h postoperatively, the median global QoR-15 score was 117 [IQR 114–121] in the ESPB group and 108 [IQR 105–111] in the control group, with a median difference of 9 points (95% CI 7–12, P < 0.001). Patients in the ESPB group scored higher on the pain, physical comfort, and emotional state subcomponents of the questionnaire. The median QoR-15 score difference at 48 h postoperatively was 3 points (95% CI 1–5, P < 0.001), with higher scores reported by patients in the ESPB group (121 [IQR 116–123]) than by patients in the control group (118 [IQR 114–122]).
Administration of single-injection ESPB before surgery reduced pain scores during the first 8 h postoperatively (P < 0.001, Fig. 2). However, the pain-sparing effect of the ESPB block disappeared 24 h after surgery. As detailed in Table 2, preoperative ESPB decreased the AUC of NRS pain scores over the initial 48 postoperative hours at rest and during mobilization (both P < 0.001). The total 24 h morphine consumption was 55% less in the ESPB group than in the control group (P < 0.001). Similarly, the median time to first rescue analgesia was significantly prolonged in patients receiving ESPB compared with no block (P < 0.001), with a difference of 6.5 h (95% CI 5.6–7.6 h). Correspondingly, preoperative bilateral ESPB compared with no block increased the patient satisfaction score by 1 point (95% CI 0–1, P < 0.001). The occurrence of PONV was significantly lower in the ESPB group than in the control group, with a relative risk of 0.38 (95% CI 0.15–0.96; P = 0.029). There was no significant difference between groups concerning pruritus and dizziness (P > 0.05). Our study identified no episodes of ESPB-related adverse events (e.g., local anesthetic systemic toxicity, bleeding, or infection).
Visualization of the distribution of pain scores using box plots at rest (A) and during mobilization (B) during the first 48 h postoperatively. Median (white dot), 25th and 75th percentiles (boxes), and range (bars) are shown. The asterisks indicate adjusted Bonferroni P < 0.05 between the two groups. NRS, numeric rating scale (range 0–10)
Discussion
Patients randomized to preoperative single-injection bilateral ESPB for lumbar spine fusion achieved a higher QoR score (indicating better quality) in the early recovery period than patients who received no block care. This trial also found that ESPB contributes significantly to improving postoperative analgesia up to 8 h, prolonging the time to rescue analgesic requirements, reducing postoperative 24 h opioid consumption, and lessening opioid-related side effects compared with no block care. Considering these findings, it is possible to conclude that preoperative administration of ESPB is a promising intervention to facilitate recovery and improve patient satisfaction after posterior lumbar interbody fusion.
We have shown that preoperative bilateral ESPB with ropivacaine provides a clinically relevant improvement in patients’ early postoperative health status. Recovery from surgery and general anesthesia is a complex process and has traditionally been measured primarily by postoperative pain intensity and opioid consumption. Recently, there has been a shift toward QoR from the patient’s perspective [14]. The QoR-15 score, a validated patient-reported outcome incorporating multiple postoperative domains, has been recommended as one of the standardized endpoints in patients following surgery [15, 16]. In this study, the global QoR-15 score 24 h postoperatively was 9 points higher in patients receiving ESPB than in general anesthesia alone. Given that a difference of 8 points in the QoR-15 score is clinically significant, our findings support the adoption of ESPB for enhanced recovery after surgery. Of note, we found a 1-point difference in the patient satisfaction score, signifying a clinically meaningful improvement. There are no data on the minimal clinically important difference (MCID) in the literature on patient satisfaction scores. We determined the MCID according to the calculation of 0.5 SD using the distribution-based method in this study. Considering that the SD from this study was 0.85 points, we defined the MCID as 0.5 points for the patient satisfaction score (range 0–10).
Given the ongoing opioid use and misuse epidemic, there is growing interest in opioid-sparing strategies in perioperative pain management [17]. Our results revealed that ultrasound-guided bilateral ESPB improved postoperative analgesia, namely, reduced AUCs of the NRS pain scores over 48 h, decreased postoperative 24 h analgesic requirements, and extended the time to first rescue analgesia, consistent with previous reports [9, 18]. However, the findings from the recently published study by Soffin et al. were not as dramatic as those presented in this manuscript [19]. A possible reason for this is that Soffin et al. employed a more robust and aggressive multimodal regimen, including ketamine, dexmedetomidine, acetaminophen, ketorolac, and intravenous lidocaine, for patients who did not receive a regional block. It is possible that this approach diminished the analgesic effect of ESPB blocks. Since only parecoxib and dexamethasone were utilized in the current study, the ESPB block might have contributed more to postoperative analgesia. Additionally, patients in the control group received approximately 60% more remifentanil, which could have led to hyperalgesia in that group, thus impacting multiple analgesic outcomes [20]. A higher degree of hyperalgesia in the nonblock group could have led to higher pain scores, higher opioid requirements, faster time to analgesic requests, and subsequent opioid-related side effects. This difference in intraoperative remifentanil administration may provide an alternative reason why the results of this study are more dramatic than those reported by Soffin et al. [19]. Our study shows that all ESPB patients required rescue analgesia by 15 h postoperatively, suggesting the block's most extended possible duration. Thus, further study is needed to identify whether a continuous local anesthetic infusion via an indwelling catheter or adjuvants added to the local anesthetic can prolong or improve analgesia. Nevertheless, if bilateral catheters were placed postoperatively, surgical manipulation might change the anatomy of the planes, leading to interference with ultrasound imaging or increased infection risk given hardware placement.
Although ESPB has been widely used as a postoperative analgesic technique with significant potential for clinical benefit, there is still controversy regarding the exact mechanism of ESPB [21]. Based on the current clinical and anatomical evidence, the direct spread and action of local anesthetics on neural targets (e.g., the dorsal rami branch, spinal nerve roots, and ventral rami branch) is the most plausible fundamental mechanism, which is in line with the clinical efficacy of ESPB—i.e., somatic analgesia, visceral analgesia, and sympathetic blockade. In this study, ESPB with ropivacaine was likely to anesthetize the dorsal rami of multiple spinal nerves by spreading within the fascial space. Anatomical and imaging research has confirmed that the dorsal rami of spinal nerves primarily innervate posterior skin, paraspinal muscles, and bony elements [22]. Thus, bilateral ESPB with ropivacaine may produce adequate analgesia covering the surgical site and relieve paraspinal muscle spasm, enhancing QoR in posterior lumbar interbody fusion patients.
The following limitations of our study should be addressed. First, we did not map the dermatomal sensory distribution of the block since ESPB was conducted under general anesthesia for blinding, consistent with our routine clinical practice. Hence, it is conceivable that there were unrecognized block failures in the ESPB group. In addition, patients could determine their treatment group given potential numbness at the surgical site. Still, the surgical team appears to have infiltrated the incisions in all patients, likely reducing this possibility. Second, the lack of NRS pain score data points between 8 and 24 h is a limitation, and the rationale for not collecting data at these time points was to avoid waking patients up in the middle of the night. Nevertheless, the time to first rescue analgesia would have provided some information regarding the clinical duration of ESPB. Third, our sample size might lack statistical power concerning secondary outcomes, such as opioid- or ESPB-related complications. Last, the generalizability of our findings may be limited because of restrictive patients from a single center.
Conclusions
In summary, single-shot preoperative bilateral ESPB reduces postoperative pain and improves the early QoR for posterior lumbar interbody fusion patients. Given the ease of performance and the theoretical safety profile, we suggest incorporating ESPB into a multimodal analgesic regimen in patients after extensive spine surgery.
References
Kim HS, Wu PH, Jang IT. Lumbar degenerative disease Part 1: anatomy and pathophysiology of intervertebral discogenic pain and radiofrequency ablation of basivertebral and sinuvertebral nerve treatment for chronic discogenic back pain: a prospective case series and review of literature. Int J Mol Sci. 2020;21:1483.
Reid PC, Morr S, Kaiser MG. State of the union: a review of lumbar fusion indications and techniques for degenerative spine disease. J Neurosurg Spine. 2019;31:1–14.
Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anaesth. 2015;62:203–18.
Neuman MD, Bateman BT, Wunsch H. Inappropriate opioid prescription after surgery. Lancet. 2019;393:1547–57.
Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152:691–7.
Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41:621–7.
Tsui BCH, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth. 2019;53:29–34.
Saadawi M, Layera S, Aliste J, Bravo D, Leurcharusmee P, Tran Q. Erector spinae plane block: a narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J Clin Anesth. 2021;68: 110063.
Zhang Q, Wu Y, Ren F, Zhang X, Feng Y. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth. 2021;68: 110090.
Singh S, Choudhary NK, Lalin D, Verma VK. Bilateral ultrasound-guided erector spinae plane block for postoperative analgesia in lumbar spine surgery: a randomized control trial. J Neurosurg Anesthesiol. 2020;32:330–4.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340: c332.
Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118:1332–40.
Myles PS, Myles DB, Galagher W, Chew C, MacDonald N, Dennis A. Minimal clinically important difference for three quality of recovery scales. Anesthesiology. 2016;125:39–45.
Myles PS. More than just morbidity and mortality—quality of recovery and long-term functional recovery after surgery. Anaesthesia. 2020;75:e143–50.
Myles PS, Boney O, Botti M, et al. Systematic review and consensus definitions for the Standardized Endpoints in Perioperative Medicine (StEP) initiative: patient comfort. Br J Anaesth. 2018;120:705–11.
Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint task force on perioperative outcome measures. Eur J Anaesthesiol. 2015;32:88–105.
Kumar K, Kirksey MA, Duong S, Wu CL. A review of opioid-sparing modalities in perioperative pain management: methods to decrease opioid use postoperatively. Anesth Analg. 2017;125:1749–60.
Finnerty D, Ní Eochagáin A, Ahmed M, Poynton A, Butler JS, Buggy DJ. A randomized trial of bilateral erector spinae plane block vs. no block for thoracolumbar decompressive spinal surgery. Anaesthesia. 2021;76:1499–503.
Soffin EM, Okano I, Oezel L, et al. Impact of ultrasound-guided erector spinae plane block on outcomes after lumbar spinal fusion: a retrospective propensity score matched study of 242 patients. Reg Anesth Pain Med. 2022;47:79–86.
Joly V, Richebe P, Guignard B, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–55.
Chin KJ, El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anaesth. 2021;68:387–408.
Bogduk N. Functional anatomy of the spine. Handb Clin Neurol. 2016;136:675–88.
Acknowledgements
We gratefully acknowledge Prof. Yusheng Yao for his support and the study participants.
Funding
The study and Rapid Service Fee was supported by Fujian Provincial Hospital.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: HL, JG, and JJ; methodology: HL, SC, and JJ; formal analysis and investigation: HL, SL, SC; writing-original draft preparation: JG and SL; writing-review and editing: JJ; resources: JJ; supervision: JJ. All authors reviewed the manuscript.
Disclosures
Huifen Lin, Jinsheng Guan, Siying Luo, Sisi Chen, and Jundan Jiang have nothing to disclose.
Compliance with Ethics Guidelines
The Ethics Committee of Fujian Provincial Hospital approved the study (no. K2019-09-026), and informed consent was obtained from all participants. The trial was conducted following the Declaration of Helsinki (revised in 2013).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lin, H., Guan, J., Luo, S. et al. Bilateral Erector Spinae Plane Block for Quality of Recovery Following Posterior Lumbar Interbody Fusion: A Randomized Controlled Trial. Pain Ther 11, 861–871 (2022). https://doi.org/10.1007/s40122-022-00395-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00395-9