Abstract
Introduction
Migraine is a common headache disorder. Many studies have used magnetic resonance imaging (MRI) to explore the possible pathogenesis of migraine, but they have not reached consistent conclusions and lack rigorous multiple comparison correction. Thus, this study investigates the mechanisms of migraine development from the perspective of altered functional connectivity (FC) in brain regions by using data-driven and regions of interest (ROI)-based approaches.
Methods
Resting-state functional MRI data were collected from 30 patients with migraine and 40 healthy controls (HCs) matched for age, gender, and years of education. For the data-driven method, we used a voxel-mirrored homotopic connectivity (VMHC) approach to compare the FC between the patients and HCs. For the ROI-based method, significant differences in VMHC maps between the patients and HCs were defined as ROI. The seed-based approach further revealed significant differences in FC between the seeds and the other brain regions. Furthermore, the correlations between abnormal FC and clinical characteristics of patients were investigated. A rigorous multiple comparison correction was used with false discovery rate and permutation test (5000 times).
Results
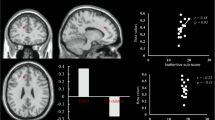
In comparison with the controls group, patients showed enhanced VMHC in the bilateral thalamus. We also observed enhanced FC between the left thalamus and the left superior frontal gyrus, and increased FC between the right thalamus and the left middle frontal gyrus (Brodmann area 45 and Brodmann area 8) in patients. Further analysis showed that the FC values in the left superior frontal gyrus and left middle frontal gyrus were negatively corrected with visual analogue scale scores or attack times for headaches.
Conclusions
Patients with migraine showed altered VMHC in the bilateral thalamus, and abnormal FC of bilateral thalamus and other brain regions. The abnormalities in thalamic FC are a likely mechanism for the development of migraine.
Trial Registration
Chinese Clinical Trial Registry, ChiCTR2000033995. Registered on 20 June 2020.
Similar content being viewed by others
Why carry out this study? |
Migraine is considered to be one of the most serious chronic dysfunctional disorders worldwide, with a high prevalence and significant loss and damage to personal health and socio-economic well-being. |
The etiology of migraine is still unclear, effective treatments are lacking, and further research into its pathogenesis is urgently needed. At present, structural MRI of patients with migraine has not revealed any problems and functional MRI (fMRI) is expected to reveal biomarkers. |
What was learned from this study? |
This study used fMRI techniques to investigate the functional connectivity differences in the brain between patients with migraine and healthy controls. Compared with healthy controls, the patients exhibited abnormal voxel-mirrored homotopic connectivity and functional connectivity in the bilateral thalamus. This may be related to the fact that the thalamus is an important sensory relay hub. |
These results provide solid evidence for the pathogenesis of migraine. In the future, studies with larger samples will be needed to further validate these findings. |
Introduction
Migraine is a common primary headache disorder characterized by recurrent pulsating moderate to severe pain attacks [1]. It can be accompanied by nausea and/or vomiting, photophobia, or phonophobia [2]. According to the latest study, migraine affects approximately 1.25 billion people worldwide [3]. The prevalence of migraine in China is 9.3% [4]. There are apparent gender differences in the incidence of migraine. It disproportionately affects women three times more than men [5]. Migraine, one of the five most chronic severe dysfunctional diseases listed by the World Health Organization (WHO), causes disability and affects the quality of life [6]. Like low back pain, old age and other hearing loss, iron deficiency anemia, and depression, migraine places a substantial socio-economic burden on society in addition to the physical and mental health toll they take on individuals [7,8,9,10,11]. The pathogenesis of migraine is still unclear, and there is no targeted treatment at present. Therefore, it is crucial to explore the mechanism of migraine.
Resting-state functional magnetic resonance imaging (rs-fMRI) has been used to explore the neurophysiological mechanisms of migraine [12,13,14,15]. Some previous studies have shown that rs-fMRI can be an effective method for studying the pathogenesis of migraine [16, 17]. Altered functional connectivity (FC) has been identified in several existing studies [12, 13, 18, 19]. Li et al. found reduced resting-state FC between the periaqueductal gray and rostral anterior cingulate cortex/medial prefrontal cortex in patients with migraine compared to healthy subjects [18]. Zhang et al. revealed that the brain regions with increased FC in the left lateral geniculate nucleus of patients with migraine compared to healthy controls (HCs) were mainly located in the left cerebellar and right lingual gyri [20]. It can be seen that the results obtained from the different tests vary relatively widely. Also, these studies suffered from a lack of rigorous correction or correction of false positives [21, 22].
The FC between the bilateral hemispheres is essential, and disruption of this connectivity may be an early change in neurological pathology [23]. Alterations in interhemispheric FC have been found in some clinical trials on neurological disorders and animal studies, both in humans and in animals [24,25,26,27,28]. Voxel-mirrored homotopic connectivity (VMHC) measures the resting-state FC between each voxel in one hemisphere and its mirror counterpart in the other hemisphere [29]. A systematic VMHC analysis provides a possible way to observe FC between the resting states of the cerebral hemispheres [30]. The different intensities of VMHC in other symmetrical regions may represent various features of interhemispheric information for bilateral sensory integration and motor coordination [31]. Previous study showed decreased VMHC of the anterior cingulate cortex (ACC) in patients with migraine without aura relative to the controls [32]. Our study is conducted to further investigate the pattern of contralateral connections in the brains of patients with migraine in order to clarify the potential pathogenesis of migraine.
In the present study, we collected rs-fMRI data from patients with migraine and healthy controls. We hypothesized that patients with migraine would have abnormal VMHC values compared to healthy controls. VMHC was performed to assess only the voxel-mirrored regions of both hemispheres. Further seed-based rs-fMRI analysis was performed to map the FC pattern between the seed region and the whole brain regions to investigate the impaired FC between the two hemispheres. We also assessed whether the clinical features of migraine were associated with altered FC in patients with migraine.
Methods
Recruitment
The study was approved by the Ethics Committee of Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine (DZMEC-KY-2020-38). Thirty right-handed patients with migraine without aura and 40 right-handed healthy subjects were recruited from the outpatient department of Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine between August 2020 and June 2021. The healthy controls had no significant differences in age, gender, or education level from the patients with migraine.
The inclusion criteria for patients with migraine were (i) age from 18 to 65 years; (ii) the patients satisfy the definition of migraine according to the International Classification of Headache Disorders, 3rd edition, beta version; (iii) no use of prophylactic treatments during the last month (including beta-blockers, calcium channel blocker, antiepileptic, antidepressant, 5-HT blocker, etc.); (iv) no use of any injectable prophylactic treatment; (v) age of the first onset less than or equal to 50 years; (vi) duration of migraine more than or equal to 1 year; (vii) at least two to no more than eight headache attacks every 4 weeks during the last 3 months. The duration of migraine is defined as how long the patient has suffered from migraine. Exclusion criteria were (i) the number of times analgesics taken for headache attacks is more than 10 per month; (ii) has taken antiepileptic or antidepressant in the last month before the study; (iii) has taken prophylactic treatments in the last month before the study; (iv) drug or alcohol abuse; (v) any other psychiatric, neurological, endocrine, cardio-cerebrovascular diseases, and other major system diseases; (vi) headache as a symptom of other diseases (e.g., hypertension, traumatic brain injury, intracranial organic disease); (vii) pregnancy or breastfeeding; (viii) any different types of headaches. All patients were asked to record in a headache diary the number of headaches, headache severity, and duration of each headache in the last month.
The inclusion criteria for HCs were (i) age from 18 to 65 years; (ii) no experience of migraine; (iii) no mental or other major systemic diseases; (iv) no MRI contraindications. The criteria for exclusion include (i) headache caused by organic disease; (ii) neurological disease, immunodeficiency, bleeding disorders, or allergy; (iii) has taken prophylactic treatments in the last 3 months before the study; (iv) drug or alcohol abuse; (v) underwent other medical examinations; (vi) pregnancy or breastfeeding; (vii) MRI contraindications.
In the present study, we obtained brain imaging data from all participants. No participants were excluded because of incomplete MRI data or other data. All participants were asked to complete a questionnaire that included demographic data, Self-rating Anxiety Scale (SAS), and Self-rating Depression Scale (SDS). In addition, patients with migraine were asked to complete duration of illness, visual analogue scale (VAS), and Migraine-Specific Quality of Life Questionnaire (MSQ, including Emotion function, Preventive, and Restrictive) [33].
MRI Data Acquisition
MRI scans were performed using a Siemens 3.0-Tesla scanner (Skyra, Siemens, Erlangen, Germany) in the Department of Radiology for Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University. Ear plugs and sponge pads were used to minimize noise exposure and head movements. During resting-state fMRI scans, subjects were asked to observe a centrally placed gaze cross (+) with their eyes open and to avoid systematic thinking as much as possible. A high-resolution t1-weighted magnetization intensity preparation of a fast gradient echo sequence was obtained and covered the entire brain [192 sagittal slices, repetition time (TR) = 2530 ms, echo time (TE) = 2.98 ms, field of view (FOV) = 240 × 240 mm, flip angle = 7°, acquisition matrix = 256 × 256, 290 scans]. Rs-fMRI data were acquired using a single-shot gradient echo-planar imaging sequence [32 axial slices, TR = 2000 ms, TE = 30 ms, FOV = 224 × 224 mm, flip angle = 90°, matrix = 256 × 256, slices thickness = 3.5 mm, voxel size 3.5 × 3.5 × 3.5 mm3].
Data Preprocessing
The functional images were preprocessed with Statistical Parametric Mapping software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) and Resting-State fMRI toolbox (DPARSF) [34]. To reduce the effect of unstable magnetization, the first ten volumes from each participant were discarded. The remaining volumes were then slice-time corrected, rearranged, and spatially normalized. Subjects whose head movements exceeded 3 mm in any direction were excluded. The images were spatially normalized into the Montreal Neurological Institute (MNI) space with a resampled voxel size of 3 × 3 × 3 mm3. After that, the data were linearly trended and band-pass filtered (0.01–0.08 Hz) to reduce the effects of low-frequency drift and high-frequency noise. The 6-mm full-width at half-maximum Gaussian kernel was used to smooth the functional images.
VMHC Analysis
Homotopic resting-state FC was calculated as the resting-state FC between any pair of symmetrical voxels between hemispheres. More specifically, in symmetrical brain space, Pearson’s correlation coefficients were calculated for the homotopic resting-state FC between each voxel’s residual time series and its symmetrical interhemispheric counterpart. Then, to obtain the normalized z-map data, Fisher’s r to z transformation was performed on the calculated correlation coefficients. The resulting values constituted the VMHC and were used in the subsequent group-level analysis.
Seed-Based FC
The seed-based FC was performed using a time-dependent process. Brain regions that showed significant differences in VMHC maps between the patients with migraine and the HCs were regions of interest (ROI). For each seed, we created whole-brain FC diagrams and tested the differences between groups of these diagrams. All seed-based FC analyses were performed using functional data sets that had been preprocessed and registered in the MNI standard space. The seeds and all symmetrical hemispheric voxels were performed using correlation analyses. To improve the normality, the correlation coefficients were switched to z values with Fisher’s r to z transformation.
Statistical Analysis
Demographic and clinical data were analyzed using SPSS statistical analysis software (version 20.0, IBM Corporation, Armonk, NY, USA). The gender distribution differences between patients with migraine and HCs were tested with the Pearson chi-square test. The independent t test was used to compare the distribution of continuous variables (age, education level). All measurement data were summarized using mean and standard deviation. P < 0.05 was considered statistically significant, and all hypothesis tests were two-tailed.
The independent t test was used to analyze VMHC comparison between groups adjusting for gender, age, and education level. The resulting P values were corrected for multiple comparisons by using permutation test (5000 times) and false discovery rate (FDR). Additionally, the VMHC value of the brain regions showed the abnormal connections between hemispheres that were extracted as ROI. For seed-based correlation analysis, the permutation test (5000 times) was performed to detect significant differences between the migraine and HCs adjusting for age, gender, and education level with the independent t test and FDR corrected. Pearson’s correlation analysis was used to detect the relationships between abnormal VMHC values or seed-based FC values and clinical characteristics in patients with migraine, including VAS, SAS, and SDS.
Results
Demographic and Clinical Characteristics
As shown in Table 1, 30 patients with migraine (mean age 36.17 ± 13.28 years, 24 women) and 40 healthy controls (mean age 36.88 ± 14.97 years, 25 women) were enrolled in the study. A total of 49 (70%) of subjects were women. There were no significant differences in age, gender, and education level between the two groups (P > 0.05). SAS and SDS were significantly different between patients and HCs (P < 0.05).
VMHC Differences Between Groups
Compared with the HCs group, the patient group showed enhanced VMHC values in the bilateral thalamus (FDR corrected p < 0.05, Fig. 1, Table 2). There were no regions that exhibited decreased VMHC values in the patient group relative to the HCs group.
Brain regions with VMHC alterations in patients with migraine. Compared with healthy controls, patients with migraine show significantly increased VHMC in bilateral thalamus. Results from two-tailed, P < 0.05, corrected by false discovery rate, permutation test 5000 times. VMHC voxel-mirrored homotopic connectivity, HC healthy controls, L left, R right, red points = patients with migraine, blue points = healthy controls
As the bilateral thalamus showed remarkable VMHC differences between the patient group and the HCs group, it was used as a biological differentiator between the two groups (Fig. 2).
ROC curves for the VMHC of bilateral thalamus in patients with migraine versus healthy controls. a AUC of right thalamus for patients vs. HCs was 0.84 ± 0.05. b AUC of left thalamus for patients vs. HCs was 0.84 ± 0.05. ROC receiver-operating characteristic, VMHC voxel-mirrored homotopic connectivity, AUC area under the receiver-operating characteristic curve
Seed-Based Functional Connectivity Between Groups
As mentioned earlier, the bilateral thalamus possessed higher VMHC values in the patient group compared to the HCs group. The seed-based FC analysis of the left thalamic seed sites revealed enhanced FC between the left thalamus and the left superior frontal gyrus (SFG) and right thalamus (FDR corrected p < 0.05, Fig. 3a, Table 3). When the right thalamus is used as a seed point correlation analysis, FC between the right thalamus and the bilateral middle frontal gyrus (MFG), which includes the left Brodmann area 8 and 45, is enhanced (FDR corrected p < 0.05, Fig. 3b, Table 3). No regions exhibited decreased FC in the patients with the HCs group.
Brain regions with FC alterations in patients with migraine. a Relative to the healthy controls, patients with migraine show increased FC between the left thalamus and superior frontal gyrus, right thalamus. b Patients with migraine show increased FC between the right thalamus and the left middle frontal gyrus, including the Brodmann area 45 and 8. Notes: Results from two-tailed, P < 0.05, corrected by false discovery rate, permutation test 5000 times. FC functional connectivity, SFG super frontal gyrus, SFG_L left super frontal gyrus, Thalamus_R right thalamus, MFG middle frontal gyrus, MFG_L left middle frontal gyrus, BA 45 Brodmann area 45, BA 8 Brodmann area 8, L left, R right, red points = patients with migraine, blue points = healthy controls
Relationships Between FC and Clinical Characteristics
As displayed in Fig. 4a, the FC values between the left thalamus and left SFG were negatively correlated with VAS (P = 0.011, r = − 0.458). In Fig. 4b, FC between the left thalamus and left SFG was negatively correlated with attack times for headaches (P = 0.034, r = − 0.388). Furthermore, the FC values between the right thalamus and the left MFG (Brodmann area 45) were negatively corrected with VAS (P = 0.012, r = − 0.455) in Fig. 4c. Also, in Fig. 4d, FC between the right thalamus and the left MFG (Brodmann area 8) was negatively correlated with attack times for headaches (P = 0.023, r = − 0.415). There was no significant correlation between the FC values and other clinical characteristics including SAS, SDS, Migraine-Specific Quality of Life Questionnaire-Emotion function (MSQE), Migraine-Specific Quality of Life Questionnaire-Preventive (MSQP), Migraine-Specific Quality of Life Questionnaire-Restrictive (MSQR), and the number of painkillers taken.
Correlation between VAS scores, attack times for headaches, and FC of the left SFG and the left MFG. a FC between the left thalamus and the left SFG was negatively corrected with VAS scores. b FC between the left thalamus and the left SFG was negatively corrected with attack times for headaches. c FC between the right thalamus and the left MFG (BA 45) was negatively corrected with VAS scores. d FC between the right thalamus and the left MFG (BA 8) was negatively corrected with attack times for headaches. Notes: Results from two-tailed, P < 0.05, Pearson correlation coefficient; FC functional connectivity, SFG super frontal gyrus, Frontal_Mid_L left middle frontal gyrus, BA 45 Brodmann area 45, BA 8 Brodmann area 8, VAS visual analogue scale
Discussion
To date, there are no specific biomarkers for migraine. The diagnosis of migraine is largely dependent on medical history and clinical symptoms. Computer tomography and conventional MRI often fail to detect any abnormalities in patients with migraine. Resting-state fMRI is considered to be a more sensitive technique for neuropsychological assessment [16, 20]. To improve our understanding of the pathogenesis of migraine, this study used resting-state fMRI methods. We found bilateral FC alterations in the thalamus of patients with migraine in the resting state. The increased FC was also found between the left thalamus and the left SFG, and right thalamus. In the right thalamus, increased FC was found with the bilateral MFG, including the left Brodmann area 45 and 8. These results revealed that the patients with migraine exhibit anomalous functional coordination between both right and left hemispheres. Further analysis showed that the FC values between the left thalamus and the left SFG were negatively corrected with VAS scores or attack times for headaches; FC between the right thalamus and the MFG were negatively corrected with VAS scores or attack times for headaches.
The thalamus is considered to be an important hub for the transmission of sensory information, transmitting received stimuli such as touch, pain, vision, and hearing to the cerebral cortex [35]. Pain is a multidimensional complex of sensations, including sensory discrimination, cognitive and emotional components. The thalamus, a critical cortical structure in the pain-related network, plays a key role in the development of pain. The thalamus is involved in the processing, perception, and modulation of pain [36]. A previous study found that pain-induced neurotransmitter transmission of endogenous opioid-activated peptides was responsible for altered thalamic blood flow and the production of nociception [37]. The blood flow and functional and structural changes of the thalamus in migraine have been reported in previous studies [38,39,40,41]. A study using arterial spin labeling methods to measure cerebral blood flow found significant relative hypoperfusion in the median region of the thalamus and significant relative hyperperfusion in the frontal cortex bilaterally during the attack phase in patients with migraine [41]. In a large multicentre study using an advanced 3-Tesla scanner, a comparison with healthy controls revealed that patients with migraine had smaller volumes of thalamic subnuclei (including the central nuclear complex, anterior nucleus, and dorsolateral nucleus) [38]. Evidence from fMRI studies have shown altered thalamic functional connectivity in patients with migraine [15, 16]. A study observed the dynamic functional connectivity of thalamocortical networks, and found that patients with migraine spent more time in a state of strong internetwork connectivity and less time in a state of sparse connectivity compared to healthy controls [42]. Another study found that people with migraine exhibited a reduction in the thalamic-pain pathway while showing an enhancement in the thalamic-visual pathway [16]. The current findings suggest significantly increased in VMHC values and enhanced FC between the bilateral thalamus, indicating a reduction in functional pain pathways in the thalamus, which may be associated with disruptions in projection, pain processing, perception, and regulation due to recurrent pain episodes. Our findings of changed function in the thalamus support previous studies. This result is valuable to further our understanding of the clinical manifestation and pathogenesis of migraine.
The SFG, located in the superior lateral frontal lobe, is a brain region responsible for emotion regulation. It is a higher center involved in the convergence and processing integration of injurious information transmitted by the internal and external nociceptive systems and has a specific role in weakening nociception [43]. Previous studies have shown that signals from the cognitive and emotional components of pain, uploaded via the medial conduction system, project via the thalamus to the prefrontal lobes and that motivated emotional responses to pain and cognitive evaluations are performed by brain regions including the prefrontal lobes [44, 45]. A study using fMRI techniques showed decreased cerebral blood flow in the right super temporal gyrus and middle frontal gyrus in patients with tinnitus and migraine [46]. Patients with migraine treated with acupuncture were found to have altered FC between the SFG and other brain regions [13]. We observed that FC between the left thalamus and the left SFG was enhanced. This provides a basis for the possible involvement of the SFG in the production of migraine.
The MFG is an important centre associated with attention. Previous research has shown that this area plays a key role in the reorientation of attention, working memory, speech, and language comprehension [47]. A study on vestibular migraine found increased FC between the left thalamus and the right MFG [16]. However, another study on migraine without aura has found reduced FC between the thalamus and the right MFG [35]. We found decreased FC between the right thalamus and the left MFG, which is consistent with previous findings.
Compared with healthy controls, we found negative corrections in the FC values between the left thalamus and the left SFG with VAS scores or attack times for headaches, and FC between the right thalamus and the left MFG was negatively corrected with VAS scores or attack times for headaches in patients with migraine. However, this correlation is positive between patients. This may be because we do not further classify migraine sufferers, or because we do not analyze the degree of pain and the duration of the illness. Detailed subgroup analyses will be needed in the future to complement our results.
Some previous studies have considered the brainstem, hypothalamus, somatosensory cortex, or visual cortex as key regions in migraine [12, 39, 40]. Our study did not find alterations in VMHC or FC in these regions. The inconsistent result may be due to that the difference in method. In some previous studies, the analytical methods were based on a priori hypotheses, and the key brain regions in pain were defined as the ROI. Different observation metrics between this study and previous studies may contribute to this inconsistent result, and in the future we will also consider using the same observation metrics to verify our results. Besides, some of the previous studies suffered from a lack of rigorous correction, and we used a more rigorous false discovery rate corrected and permutation test (5000 times).
As with all the studies, the current research needs to be considered with several potential limitations. First, our study is cross-sectional research, and the causal relationship between the brain changes and migraine is still unclear. Second, the sample size in our study is small, but we have performed a rigorous calibration and future studies with larger samples could further validate our results. Third, we did not record the time from the scan to the previous attack in patients with migraine. Some studies requested a scan 48 or 72 h after a migraine attack [35, 48]. In future, in order to further improve our study, we will more strictly limit the time in which patients receive scans. Fourth, we adopted the whole thalamus as the seeds in the current study; however, different subregions of the thalamus may have particular functions. Therefore, further research is needed to understand better the correlation between other regions of the thalamus and the pathogenesis of migraine.
Conclusion
Overall, although our findings need to be validated using larger sample size, the present results reveal that abnormalities in thalamic functional connectivity are a likely mechanism for the development of migraine. Resting-state fMRI may be an essential diagnostic modality for differentiating migraine from other types of headaches. Scientific explanations of the underlying mechanisms of migraine need increasing attention.
References
Seo J, Chu H, Kim CH, Sung KK, Lee S. Cupping therapy for migraine: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2021;2021:7582581.
Ashina M. Migraine. N Engl J Med. 2020;383(19):1866–76.
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858.
Yu S, Liu R, Zhao G,et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache. 2012;52(4):582–91.
Lagman-Bartolome AM, Lay C. Migraine in women. Neurol Clin. 2019;37(4):835–45.
Hay SI, Abajobir AA, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260–344.
Kiely KM, Gopinath B, Mitchell P, Luszcz M, Anstey KJ. Cognitive, health, and sociodemographic predictors of longitudinal decline in hearing acuity among older adults. J Gerontol A Biol Sci Med Sci. 2012;67(9):997–1003.
Pasricha SR, Drakesmith H, Black J, Hipgrave D, Biggs BA. Control of iron deficiency anemia in low- and middle-income countries. Blood. 2013;121(14):2607–17.
Schilder AG, Chong LY, Ftouh S, Burton MJ. Bilateral versus unilateral hearing aids for bilateral hearing impairment in adults. Cochrane Database Syst Rev. 2017;12:CD012665.
Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–66.
O’Keeffe M, O‘Sullivan P, Purtill H, Bargary N, O’Sullivan K. Cognitive functional therapy compared with a group-based exercise and education intervention for chronic low back pain: a multicentre randomised controlled trial (RCT). Br J Sports Med. 2020;54(13):782–9.
Li Z, Zeng F, Yin T, et al. Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. Neuroimage Clin. 2017;15:367–75.
Zou Y, Tang W, Li X, Xu M, Li J. Acupuncture reversible effects on altered default mode network of chronic migraine accompanied with clinical symptom relief. Neural Plast. 2019;2019:5047463.
Arngrim N, Hougaard A, Schytz HW, et al. Effect of hypoxia on BOLD fMRI response and total cerebral blood flow in migraine with aura patients. J Cereb Blood Flow Metab. 2017;39(4):680–9.
Chang CM, Yang CP, Yang CC, Shih PH, Wang SJ. Evidence of potential mechanisms of acupuncture from functional MRI data for migraine prophylaxis. Curr Pain Headache Rep. 2021;25(7):49.
Chen Z, Xiao L, Liu H, et al. Altered thalamo-cortical functional connectivity in patients with vestibular migraine: a resting-state fMRI study. Neuroradiology. 2021;64(1):119–27.
Tu Y, Zeng F, Lan L, et al. An fMRI-based neural marker for migraine without aura. Neurology. 2020;94(7):e741–51.
Li Z, Liu M, Lan L, et al. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 2016;6:20298.
Li K, Zhang Y, Ning Y, et al. The effects of acupuncture treatment on the right frontoparietal network in migraine without aura patients. J Headache Pain. 2015;16:518.
Zhang D, Huang X, Su W, et al. Altered lateral geniculate nucleus functional connectivity in migraine without aura: a resting-state functional MRI study. J Headache Pain. 2020;21(1):17.
Planchuelo-Gomez A, Garcia-Azorin D, Guerrero AL, et al. Alternative microstructural measures to complement diffusion tensor imaging in migraine studies with standard MRI acquisition. Brain Sci. 2020;10(10):711.
Seminowicz DA, Burrowes SAB, Kearson A, et al. Enhanced mindfulness-based stress reduction in episodic migraine: a randomized clinical trial with magnetic resonance imaging outcomes. Pain. 2020;161(8):1837–46.
Guo S, Kendrick KM, Zhang J, et al. Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression. Neuroimage Clin. 2013;2:818–26.
Wang W, Peng Z, Wang X, et al. Disrupted interhemispheric resting-state functional connectivity and structural connectivity in first-episode, treatment-naïve generalized anxiety disorder. J Affect Disord. 2019;251:280–6.
van Meer MPA, van der Marel K, Otte WM, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Correspondence between altered functional and structural connectivity in the contralesional sensorimotor cortex after unilateral stroke in rats: a combined resting-state functional MRI and manganese-enhanced MRI study. J Cereb Blood Flow Metab. 2010;30(10):1707–11.
Siegel JS, Ramsey LE, Snyder AZ, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci U S A. 2016;113(30):E4367–76.
Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63(2):236–46.
Javor-Duray BN, Vinck M, van der Roest M, et al. Alterations in functional cortical hierarchy in hemiparkinsonian rats. J Neurosci. 2017;37(32):7669–81.
Zuo XN, Kelly C, Di Martino A, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30(45):15034–43.
Xu Q, Zhang Z, Liao W, et al. Time-shift homotopic connectivity in mesial temporal lobe epilepsy. AJNR Am J Neuroradiol. 2014;35(9):1746–52.
Stark DE, Margulies DS, Shehzad ZE, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28(51):13754–64.
Yuan K, Qin W, Liu P, et al. Reduced fractional anisotropy of corpus callosum modulates inter-hemispheric resting state functional connectivity in migraine patients without aura. PLoS ONE. 2012;7(9):e45476.
Chang HY, Jensen MP, Yang CC, Lai YH. Migraine-Specific Quality of Life Questionnaire Chinese version 2.1 (MSQv2.1-C): psychometric evaluation in patients with migraine. Health Qual Life Outcomes. 2019;17(1):108.
Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13.
Qin ZX, Su JJ, He XW, et al. Altered resting-state functional connectivity between subregions in the thalamus and cortex in migraine without aura. Eur J Neurol. 2020;27(11):2233–41.
Schwedt TJ, Schlaggar BL, Mar S, et al. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache. 2013;53(5):737–51.
Bogdanov VB, Vigano A, Noirhomme Q, et al. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fMRI study in healthy subjects. Behav Brain Res. 2015;281:187–98.
Magon S, May A, Stankewitz A, et al. Morphological abnormalities of thalamic subnuclei in migraine: a multicenter MRI Study at 3 Tesla. J Neurosci. 2015;35(40):13800–6.
Wei HL, Zhou X, Chen YC, et al. Impaired intrinsic functional connectivity between the thalamus and visual cortex in migraine without aura. J Headache Pain. 2019;20(1):116.
Chong CD, Aguilar M, Schwedt TJ. Altered hypothalamic region covariance in migraine and cluster headache: a structural MRI study. Headache. 2020;60(3):553–63.
Kato Y, Araki N, Matsuda H, Ito Y, Suzuki C. Arterial spin-labeled MRI study of migraine attacks treated with rizatriptan. J Headache Pain. 2010;11(3):255–8.
Tu Y, Fu Z, Zeng F, et al. Abnormal thalamocortical network dynamics in migraine. Neurology. 2019;92(23):e2706–16.
Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(Pt 5):1079–91.
Woo CW, eSchmidt L, Krishnan A, et al. Quantifying cerebral contributions to pain beyond nociception. Nat Commun. 2017;8:14211.
Cauda F, Torta DM, Sacco K, et al. Shared “core” areas between the pain and other task-related networks. PLoS ONE. 2012;7(8):e41929.
Xu ZG, Xu JJ, Chen YC, et al. Aberrant cerebral blood flow in tinnitus patients with migraine: a perfusion functional MRI study. J Headache Pain. 2021;22(1):61.
Briggs RG, Lin YH, Dadario NB, et al. Anatomy and white matter connections of the middle frontal gyrus. World Neurosurg. 2021;150:e520–9.
Kim YE, Kim MK, Suh SI, Kim JH. Altered trigeminothalamic spontaneous low-frequency oscillations in migraine without aura: a resting-state fMRI study. BMC Neurol. 2021;21(1):342.
Acknowledgements
We thank the Radiology Department of Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University for their support. We thank all participants for their involvement in this study.
Funding
The study was funded by National Natural Science Foundation of China Youth Fund (82004197), Beijing Excellent Talent Training Project (2018000020124G112) and Project for new teachers of Beijing University of Chinese Medicine (2020-JYB-XJSJJ-045). Publication fees (include the Rapid Service Fee and Open Access Fee) were provided by National Natural Science Foundation of China Youth Fund (82004197).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
We fully acknowledge all the investigators and clinical staff in the study for their efforts. Zi-Min Cao, Jian-Wei Huo, Chao-qun Yan, and Jun Wang contributed to the concept and design of the study. Zi-Min Cao, Yi-Chao Chen, and Guo-Yun Liu recruited participants and collected data. An-Qi Shi, Lu-Fan Xu, and Zhi-Jun Li were responsible for the statistics and analysis of clinical data on subjects. Ya-Nan Zhang, Ni Liu and Jian-Wei Huo were responsible for scanning fMRI. Zi-Min Cao, Xu Wang and Jun Wang contributed to the drafting of the manuscript and discussions. Zi-Min Cao, Xu Wang, Chao-Qun Yan, and Jun Wang revised the manuscript is critical. Jun Wang supervised the study. All authors approved the final version of the manuscript.
Disclosures
All authors (Zi-Min Cao, Yi-Chao Chen, Guo-Yun Liu, Xu Wang, An-Qi Shi, Lu-Fan Xu, Zhi-Jun Li, Ya-Nan Zhang, Ni Liu, Jian-Wei Huo, Chao-Qun Yan, and Jun Wang) confirm that they have no financial or other conflicts of interest to disclose.
Compliance with Ethics Guidelines
The study was approved by the Ethical Committee of Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine (DZMEC-KY-2020-38). The trial was performed in accordance with the Declaration of Helsinki. All researchers protected the health and rights of each participant. Before the study, each participant received informed consent.
Data Availability
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cao, ZM., Chen, YC., Liu, GY. et al. Abnormalities of Thalamic Functional Connectivity in Patients with Migraine: A Resting-State fMRI Study. Pain Ther 11, 561–574 (2022). https://doi.org/10.1007/s40122-022-00365-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00365-1