Abstract
Introduction
Most current treatment strategies and investigations on cryptococcal meningitis (CM) focus primarily on the central nervous system (CNS), often overlooking the complex interplay between the CNS and the peripheral system. This study aims to explore the characteristics of central and peripheral metabolism in patients with CM.
Methods
Patients diagnosed with CM as per the hospital records of the Fourth People’s Hospital of Nanning were retrospectively analyzed. Patients were divided into two groups, non-structural damage of the brain (NSDB) and structural damage of the brain (SDB), according to the presence of brain lesions as detected with imaging. Based on the presence of enlarged cerebral ventricles, the cases in the SDB group were classified into non-ventriculomegaly (NVM) and ventriculomegaly (VM). Various parameters of cerebrospinal fluid (CSF) and peripheral blood (PB) were analyzed.
Results
A significant correlation was detected between CSF and PB parameters. The levels of CSF-adenosine dehydrogenase (ADA), CSF-protein, CSF-glucose, and CSF-chloride ions were significantly correlated with the levels of PB-aminotransferase, PB-bilirubin, PB-creatinine (Cr), PB-urea nitrogen, PB-electrolyte, PB-protein, and PB-lipid. Compared with NSDB, the levels of CSF-glucose were significantly decreased in the SDB group, while the levels of CSF-lactate dehydrogenase (LDH) and CSF-protein were significantly increased in the SDB group. In the SDB group, the levels of PB-potassium, PB-hemoglobin(Hb), and PB-albumin were significantly decreased in the patients with VM, while the level of PB-urea nitrogen was significantly increased in these patients.
Conclusion
Metabolic and structural alterations in the brain may be associated with peripheral metabolic changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Cryptococcal meningitis (CM) which imposes an enormous burden on patients, families, and society will result in serious consequences of central nervous system (CNS) and peripheral system (also clarified as peripheral organ systems). |
Most current treatment strategies and investigations often overlook the complex interplay between the CNS and the peripheral system. |
What was the hypothesis of the study? |
The lesions of CNS (including metabolic disorders followed by structural damage) may affect the energy metabolism of peripheral systems. |
What were the study outcomes/conclusions? |
Metabolic and structural alterations in the brain may be associated with peripheral metabolic changes in patients with CM. |
What has been learned from the study? |
Timely detection of metabolic changes in the CNS to avoid structural damage in the CNS. |
In addition to paying attention to CNS lesions, it is necessary to consider the impact that CNS lesions may have on the peripheral system, especially the systemic imbalance of energy metabolism. |
Introduction
Cryptococcal meningitis (CM) is an infectious disease of the central nervous system (CNS) caused by the fungal pathogen Cryptococcus neoformans. The disease has a very high mortality rate of 83.30–90.00% [1,2,3]. C. neoformans first invades the pia, and, with the progression of the disease, it gradually invades the brain parenchyma and may even affect the cerebrovascular and cerebral ventricles [4]. It can also cause functional disorders of the CNS, resulting in various neurological defects, such as headaches, seizures, changes in consciousness, and limb motor dysfunction [5], which impose an enormous burden on patients, families, and society [6].
Previous studies have identified two stages of pathogenic microorganism-induced CNS damage, where the first stage involves metabolic disorders [7,8,9], while the second involves structural lesions [10]. As per the results of previous experiments, structural damage of the brain was observed in 86.84–90.00% of patients with CM, while no structural damage of the brain was observed in the remaining patients [11, 12]. Zhong et al. demonstrated that the brain structure of patients with CM was normal in the early stage, manifesting only a few symptoms of nerve damage, followed by gradually appearing imaging changes, namely, structural damage [11]. This suggested that the effects of C. neoformans on the brain also progress from early non-structural damage of the brain to structural damage. Tu et al. found that patients with CM with structural damage of the brain had a higher percentage of poor prognosis [13]. Studies have consistently shown that patients with structural damage of the brain not only have neurological disorders but also may have damage to multiple organ systems throughout the body [14,15,16].
Accumulating evidence suggests that complex interaction exists between CNS and other systems, where the brain acts as the higher regulatory center, and the autonomic nervous system (ANS) is the final effector in regulating the activities of other systems, particularly the energy metabolism of peripheral systems (also clarified as peripheral organ systems) [17,18,19]. While the brain is diseased, the normal neuroregulatory network is affected, resulting in changes in the peripheral system [16, 20,21,22,23]. Most current treatment strategies and investigations on CM focus primarily on the CNS, often overlooking the complex interplay between the CNS and the peripheral system.
Energy metabolism homeostasis is an essential requirement to maintain a biological process [19, 24], and is regarded as a key factor affecting the prognosis of patients with CNS lesions [25,26,27]. Energy metabolism homeostasis mainly includes the metabolism of the CNS and that of the peripheral system. In addition, lesions in the CNS may cause metabolic abnormalities in the peripheral system, leading to malnutrition, such as electrolyte disconcert, abnormal lipid metabolism, and abnormal protein metabolism [28,29,30]. Even worse, peripheral metabolic disorders can lead to CNS damage [31]. Our previous study confirmed that the disruption of glucose metabolism predated neuronal damage [32], which suggested that correcting the disruption of energy metabolism may save neurons and avoid damage to multiple organ systems. Therefore, maintaining central/peripheral metabolic homeostasis for patients with CM is extremely important to protect the nervous system and to improve their prognosis. Nevertheless, there is a paucity of data on central/peripheral metabolic changes in patients with CM, which makes their treatment challenging. In light of this challenge, we conducted a retrospective analysis of 120 patients with CM, who were divided into two groups: non-structural damage of the brain (NSDB) and structural damage of the brain (SDB) via brain imaging. We compared the differences in cerebrospinal fluid (CSF) and peripheral blood (PB) between different groups to provide theoretical guidance for CM treatment.
Methods
Study Design
In this retrospective study, patients diagnosed with CM as per the hospital records of the Fourth People’s Hospital of Nanning from January 2019 to December 2022 were selected. The hospital Institutional Ethics Committee reviewed and approved the study protocol. The Ethics Committee of the Fourth People’s Hospital of Nanning waived patient informed consent because we used anonymous patient electronic medical records in this study. The study was in accordance with the Declaration of Helsinki. This study was approved by the Fourth People’s Hospital of Nanning and registered under project number [2023] 69.
Patient Enrollment and Diagnostic Criteria for CM
All enrolled patients had CM, and the definitive diagnosis was based on a positive CSF test result, including the isolation of C. neoformans in CSF cultures, a positive cryptococcal antigen in CSF, or a positive ink stain in CSF. Cases with incomplete neuroimaging or cerebrospinal fluid data and cases with a history of metabolic disease (such as diabetes, hyperthyroidism, Cushing’s syndrome, primary hyperaldosteronism, etc.) were excluded. According to the above criteria, we obtained the data from 120 patients with CM.
Demographic and Clinical Data Collection
We conducted a retrospective analysis of 120 patients with CM, including gender, age, height, weight, treatment regimen, results of CSF analysis, and peripheral blood PB analysis. The results of CSF analysis included white cell counts, glucose, adenosine dehydrogenase (ADA), lactate dehydrogenase (LDH), chloride ion (Cl), and proteins. The results of PB analysis included glucose, hemoglobin (Hb), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), direct bilirubin (DBIL), total protein, globulin, albumin (ALB), creatinine (Cr), urea, uric acid (UA), potassium (K), sodium (Na), calcium (Ca), chloride ion (Cl), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Based on the results of brain imaging, patients with CM with normal brain imaging were a part of the NSDB group, and those with abnormal brain imaging were a part of the SDB group. SDB was defined as CM-related brain damage.
Statistical Analysis
SPSS 25.0 was used for statistical analysis. The correlation between the data was estimated using Pearson’s correlation coefficient. An independent-sample t test was conducted to assess the significance of the differences between the two data groups. The data are expressed in terms of the mean ± standard deviation. A P value < 0.05 was regarded as statistically significant.
Results
Population Characteristics
The characteristics of the patient population are summarized in Table 1. There were 92 males and 28 females, aged 48.66 ± 13.88 years, with height 163.64 ± 6.65 cm, weight 54.23 ± 10.11 kg, and BMI 20.18 ± 3.21. The prognosis of all 120 patients with CM was as follows: cured patients, 1; improved patients, 97; not improved patients, 10; patients who died, 13. A total of 118 patients received antifungal therapy, with the main drugs being Voriconazole (VRC), Fluorocytosine (FC), Fluconazole (FCZ), Itraconazole (ITRA), and Amphotericin B (AmpB).
Imaging Characteristics of the Brain
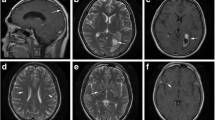
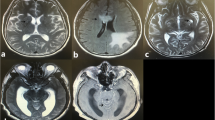
There were 48 patients in the NSDB group and 72 in the SDB group. The patients in the NSDB group were characterized by normal brain imaging (Fig. 1A). The patients in the SDB group were primarily characterized by CM-related brain damage(Fig. 1B). The characteristics of the NSDB and SDB groups are summarized in Table 2. In patients with CM alone, the BMI of patients in the SDB group was significantly lower than that in the NSDB group (Table 2).
MRI in NSDB and SDB groups: A MRI in NSDB group; B MRI in SDB group (NVM); C MRI in SDB group (VM); red arrows cryptococcal meningitis-related brain damage, yellow arrows ventriculomegaly. MRI magnetic resonance imaging; NSDB non-structural damage of the brain; SDB structural damage of the brain; NVM non-ventriculomegaly; VM ventriculomegaly
Biochemical Characteristics of CSF and Peripheral Blood
We detected a correlation between CSF and PB characteristics (Table 3). In all patients, the levels of CSF-ADA were negatively correlated with the levels of PB-ALT and PB-Cl. The levels of CSF-protein were negatively associated with the levels of PB-ALT, PB-Cr, PB-UA, and PB-Cl, but positively linked to that of PB-HDL-C. The levels of CSF-glucose were positively correlated with the levels of PB-TBIL, PB-urea, PB-Cr, PB-UA, PB-Na, PB-Cl, and PB-TG. The levels of CSF-Cl pairs were significantly positively associated with the levels of PB-ALB, PB-TBIL, PB-DBIL, PB-UA, PB-Na, and PB-Cl, but significantly negatively linked to the levels of PB-HDL-C. Subsequently, we respectively detected a correlation between CSF and PB characteristics in the NSDB and SDB groups. In the NSDB group, the levels of CSF-ADA were negatively correlated with the levels of PB-Na and PB-Cl, but positively linked to the levels of PB-TBIL. The levels of CSF-glucose were positively correlated with the levels of PB-urea and PB-TG. The levels of CSF-Cl were positively linked to the levels of PB-Na and PB-Cl, but negatively associated with the levels of PB-AST and PB-ALT. In the SDB group, the levels of CSF-ADA were negatively correlated with PB-AST and PB-ALT. The levels of CSF-protein were negatively linked to the levels of PB-AST, PB-ALT, and PB-Cl, but positively associated with the levels of PB-TC and PB-LDL-C. The levels of CSF-Cl were positively correlated with the levels of PB-ALB, PB-TBIL, PB-UA, PB-Na, and PB-Cl, but negatively linked to PB-HDL-C.
The results of the analysis of the characteristics of CSF and PB in the NSDB and SDB groups are summarized in Table 4. Compared with the NSDB group, the levels of CSF-glucose were significantly decreased in the SDB group (P < 0.05), and the levels of CSF-LDH (P < 0.05) and CSF-protein (P < 0.01) were significantly increased in the SDB group (P < 0.05). Based on the imaging of the brain, the difference in CSF and PB characteristics between NSDB and SDB in patients with CM alone, CM combined with TB, CM combined with HIV, and CM combined with HIV and TB are specified in Table 5. In patients with CM alone, the levels of CSF-glucose in the SDB group were significantly lower than those in the NSDB group (P < 0.05), and the levels of PB-K were higher than those in the NSDB group (P < 0.05).
Characteristics of Patients with Alterations in Cerebral Ventricles
In the SDB group, there were 27 patients with and ventriculomegaly (VM; Fig. 1C). The patients in the SDB group were divided into the non-VM (NVM) and VM groups. The general characteristics of patients in these groups are enumerated in Table 5. The age of patients in the NVM group (45.27 ± 12.45 years) was significantly lower than that in the VM group (55.89 ± 14.30 years; P < 0.01). The results of the analysis of the characteristics of CSF and PB in these two groups are summarized in Table 6. Compared with the NVM group, the levels of PB-Hb (P < 0.05), PB-ALB (P < 0.05), and PB-K (P < 0.01) were significantly decreased in the VM group, and the levels of PB-UA (P < 0.05) were significantly increased in the VM group. Of note, hypokalemia is recognized as a common side effect of AmpB [33]. To ascertain whether AmpB led to alterations in the peripheral metabolism, the patients in the SDB group were divided into two groups, namely, the non-received AmpB group and the received AmpB group. The results revealed no significant difference in the levels of PB-K, PB-Hb, PB-ALB, and PB-UA (Table 7).
Discussion
C. neoformans may lead to astrocyte proliferation, microglial activation, and neuronal apoptosis after its entry into the CNS via direct transmembrane transport, disruption of the blood–brain barrier, and “Trojan horse”(dissemination to the brain by indirect carriage within host mononuclear phagocytes) [34,35,36,37]. During this process, the brain is observed to have a significant inflammatory response, which can lead to brain damage [38]. The first manifestation of brain damage is metabolic disorders, followed by structural damage [10, 39,40,41]. After an invasion of the CNS by pathogenic microorganisms, the patients with CNS infectious diseases may experience some symptoms [7], including seizures, abnormal mental status, apraxia, etc., although there is no significant observable loss of neurons or other substantial pathological changes detected in those patients during this stage [8, 42]. Previous studies have suggested that the abnormal levels of glucose and adenosine triphosphate (ATP) in brain were observed during this stage [43,44,45].Before the structural damage of the brain was observed, the concentrations of CSF-glucose in some individuals with CM were found to be lower than the normal level, which seemed to indicate that these individuals already have a disorder of brain energy metabolism [11]. As the disease advances, the patients with CNS infectious diseases often experience worsening motor dysfunction and cognitive impairments, and structural damage of the brain may be observed, such as basal meningeal enhancement, hydrocephalus, or cryptococcoma [46]. Moreover [47], a brain with structural damage may also be accompanied by severe metabolic disorders [46, 48]. The normal central–peripheral regulatory network may be affected by brain damage, resulting in changes in the peripheral system [16, 20,21,22,23]. We found that the levels of CSF metabolites in patients with CM were correlated with electrolytes, proteins, lipids, and metabolites of liver and kidney function in peripheral blood. Even in the NSDB group, there was a significant correlation between the CNS and the levels of metabolites in the peripheral system, which also suggested that the disorder of CNS metabolism may lead to a disorder of peripheral metabolism. These patients with CM with a normal brain structure also progress to the second stage of brain structural damage [11]. Based on the imaging of the brain, we found that patients with CM with structural damage of the brain accounted for 60%, while patients with CM with non-structural damage of the brain accounted for 40%. This result is fairly different from the studies of Zhong et al. [11] and Anjum et al. [12], possibly due to regional and population differences. The levels of CSF-protein are considered an indicator of the severity of inflammation in the brain [49]. We found that the level of CSF-protein in the SDB group was significantly higher, which suggested that the more severe the inflammatory response, the more likely the brain structural changes. In addition, Yang et al. demonstrated that patients with stroke were observed the elevated protein levels in CSF, suggesting that structural damage may lead to higher level of protein in CSF [50]. Our results also suggested that the structural damage leading from C. neoformans may lead to higher protein elevation. The long-term disturbance of energy metabolism in the CNS may be a crucial factor leading to structural damage [32]. The energy utilization of the brain is mainly dependent on ATP, which mainly comes from glucose [51, 52]. The decreased concentration of CSF-glucose is considered an independent prognostic factor, which represents an unsatisfactory outcome in patients with CM [53]. After the invasion of the brain, C. neoformans leads to a decrease in the concentration of CSF-glucose, resulting in an insufficient energy supply of cells, which eventually damages neurons and gradually leads to structural changes in the brain [54]. There are several reasons for the decreased concentration of glucose in the brain caused by C. neoformans. When invading the brain, C. neoformans may destroy the blood–brain barrier, especially the functional structures of endothelial cells, which can create obstacles in the glucose’s access to the brain [55]. Astrocyte–neuron metabolic coupling is the primary mechanism of brain energy metabolism [56]. The astrocyte proliferation induced by C. neoformans may lead to changes in the glucose levels of the brain [34]. In addition, C. neoformans reproduction takes in a large amount of glucose from the outside, resulting in its decreased levels in the brain [57]. Zhang et al. suggested that the lower the level of CSF glucose, the worse the prognosis of patients with CM [58]. Considering the importance of CSF glucose levels, we compared the CSF-glucose levels of patients in the NSDB group and the SDB group, and the results revealed that the CSF-glucose levels in the SDB group were lower. This result also suggested that decreased levels of cerebral glucose are associated with structural brain damage in patients with CM, and that levels of CSF glucose may be regarded as a crucial indicator of possible structural damage. Therefore, in the face of patients with CM, we not only need to carry out antifungal treatment for the patients but also need to pay attention to the levels of CSF glucose and timely correction of CNS glucose metabolism disorders and avoid structural brain damage. In addition to CSF-glucose, the CSF/blood glucose (reference value 50–70%), is considered as an indicator of the prognosis of CNS infectious disease [59,60,61]. Previous studies suggested that the low CSF/blood glucose ratio was associated with poor outcomes, including seizures, deep coma, or death [61, 62]. Zheng et al. demonstrated that CSF/blood glucose ratio was the factor significantly related to the survival time in patients with CM with acute/subacute onset [63]. However, we found no significant difference in the CSF/blood glucose ratio between the NSDB group and the SDB group. The result of the CSF/blood glucose ratio differs from Zhong et al., possibly due to differences in sample size and inclusion criteria [11]. There was no significant difference in mortality between the NSDB group and the SDB group, which was consistent with Zhong et al [11], suggesting that structural damage of the brain may not affect the survival of patients with CM.
Besides the alteration in the CNS glucose metabolism, the lactate metabolism in the CNS must also be considered. Previous studies have demonstrated that abnormal lactate metabolism in patients with CM brain are closely related to poor prognosis and can also lead to serious consequences such as abnormal mental state, seizures, and significant increase in intracranial pressure [46, 64, 65]. In this study, higher levels of CSF-LDH were observed in the SDB group, which suggested that the abnormal lactate metabolism of the CNS may be related to the structural damage of the brain. Astrocyte–neuron coupling is the chief mechanism of lactate metabolism in the brain [66]. When vascular endothelial cells transfer glucose from peripheral blood to the CNS, astrocytes absorb glucose into the cells and then gradually transform glucose into pyruvate through aerobic fermentation, participating in the tricarboxylic acid cycle to produce ATP, and some pyruvic acid is converted into lactate by LDH5, one of the subtypes of LDH [16, 67]. Astrocytes lactate is transported from the intracellular to extracellular by monocarboxylate transporter 4 (MCT4), which forms a “lactate pool” between the astrocytes and neurons [68, 69]. The lactate from the lactate pool is transported to neurons by monocarboxylate transporter 2 (MCT2) [16, 70, 71]. The lactate that enters the neuron is converted into pyruvate under the action of LDH1, another subtype of LDH, which participates in the tricarboxylic acid cycle of the neuron and produces ATP [66, 70, 72]. The levels of LDH1 and LDH5 determine the efficiency of the conversion of lactate to pyruvate and regulate the levels of lactate in the brain. Nussinovitch et al. found that infection of the brain by pathogenic microorganisms could lead to a decrease in the proportion of CSF-LDH1 and an increase in the proportion of CSF-LDH5 [73]. We noticed an increase in the levels of CSF-LDH, but could not obtain the data of CSF-LDH5 and CSF-LDH1.We speculate that there may also be a decrease in LDH1 and/or an increase in LDH5 in the CSF of patients with CM. However, this hypothesis needs to be corroborated by more experimental data.
In the SDB group, we noted that patients with CM with VM were older, an observation similar to that made by Kellogg et al. [74], suggesting that VM may be age-related. However, we were more interested in the presence of significant peripheral metabolic abnormalities in patients with CM with VM. The levels of PB-UA were significantly increased in patients with VM, while the levels of PB-K, PB-Hb, and PB-ALB were significantly decreased, suggesting that these patients may have abnormal energy metabolism and poor nutritional status, which would affect the prognosis of neurological diseases [75, 76]. The most common peripheral metabolic change in patients with CM is hypokalemia, which is also a difficult and major challenge in the treatment of CM [33, 77, 78]. Hypokalemia is often associated with renal toxicity caused by AmpB [33]. However, we compared the levels of PB-Cr between the NVM and VM groups and detected no significant difference, which does not support changes in blood potassium due to renal toxicity. To determine whether AmpB is associated with hypokalemia, patients with CM were divided into two groups: those who did not use AmpB and those who used AmpB. The results revealed no significant difference in serum potassium concentrations between the two groups, which suggested that AmpB is not a major factor for the disorder of peripheral metabolism. Based on our results, we conclude that VM may be the main factor leading to abnormal peripheral energy metabolism.
It is well known that the hypothalamus is an important brain region responsible for regulating energy metabolism throughout the body, including energy intake, energy expenditure, and energy storage [19, 79]. The glucose-sensing neurons (GSNs) in the hypothalamus play a key role in energy intake, energy expenditure, and energy storage [80]. When GSNs receive brain glucose signals, the hypothalamus outputs signals through the ANS to regulate pancreatic function and gastrointestinal function to achieve the balance of body energy metabolism [81, 82]. When glucose levels in the brain are reduced, GSNs inhibit anorexic behavior, activate gastrointestinal neurons, increase energy intake, and reduce energy expenditure [83]. However, the hypothalamus does not contact CSF directly. Instead, it senses changes in CSF-glucose levels by receiving signals from tanycytes located in the cerebral ventricles [84]. Tanycytes play an important role in energy metabolism [85], and disruption of their function can lead to abnormalities in food intake, fat mass, and fatty acid oxidation [86]. Since the CSF glucose level is low in patients with CM with structural damage of the brain, VM may disrupt the function of tanycytes and affect glucose signaling in the hypothalamus, resulting in insufficient energy intake and excessive energy consumption, as well as decreased levels of PB-K, PB-Hb, and PB-ALB and increased levels of PB-urea.
Taken together, we observed that the metabolic and structural changes in CNS in patients with CM are related to the energy metabolism of the peripheral system. However, due to the lack of data in some cases and the shortage of the study sample, this research has a few limitations, which should be acknowledged. There is no direct evidence to confirm whether the disruption of central energy metabolism caused by C. neoformans affects peripheral energy metabolism. The research was based on a retrospective analysis, and further clinical or experimental evaluation should be necessary to verify the causal relationship between CNS changes and peripheral system changes in CM. Meanwhile, it is necessary to build reliable in vitro and in vivo models to identify the potential mechanisms of CNS regulation of peripheral system energy metabolism in CM.
Conclusions
We have found that structural damage of the brain may be related to abnormal CNS energy metabolism and further revealed that VM might be related to peripheral energy metabolism disorders. This study provides two ideas for us to face CNS diseases in the future: (1) timely detection of metabolic changes in the CNS to avoid structural damage in the CNS, and ( 2) in addition to paying attention to CNS lesions, it is necessary to consider the impact that CNS lesions may have on the peripheral system, especially the systemic imbalance of energy metabolism.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fisher KM, Montrief T, Ramzy M, Koyfman A, Long B. Cryptococcal meningitis: a review for emergency clinicians. Intern Emerg Med. 2021;16:1031–42.
Elsegeiny W, Marr KA, Williamson PR. Immunology of cryptococcal infections: developing a rational approach to patient therapy. Front Immunol. 2018;9:651.
Rajasingham R, Smith RM, Park BJ, et al. global burden of disease of HIV-associated cryptococcal meningitis an updated analysis. Lancet Infect Dis Actions Search in PubMed Search in NLM Catalog Add to Search. 2017;17:873–81.
Sarkis RA, Mays M, Isada C, Ahmed M. MRI findings in cryptococcal meningitis of the non-HIV population. Neurologist. 2015;19:40–5.
Makadzange AT, McHugh G. New approaches to the diagnosis and treatment of cryptococcal meningitis. Semin Neurol. 2014;34:47–60.
Dromer F, Mathoulin-Pélissier S, Launay O, Lortholary O. French Cryptococcosis Study Group Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4(2): e21.
Kaku M, Simpson DM. HIV neuropathy. Curr Opin HIV AIDS. 2014;9:521–6.
Seilhean D, Duyckaerts C, Vazeux R, et al. HIV-1-associated cognitive/motor complex: absence of neuronal loss in the cerebral neocortex. Neurology. 1993;43:1492–9.
Samboju V, Philippi CL, Chan P, et al. Structural and functional brain imaging in acute HIV. Neuroimage Clin. 2018;20:327–35.
Tater P, Pandey S. Post-stroke movement disorders: clinical spectrum, pathogenesis, and management. Neurol India. 2021;69:272–83.
Zhong Y, Zhou Z, Fang X, Peng F, Zhang W. Magnetic resonance imaging study of cryptococcal neuroradiological lesions in HIV-negative cryptococcal meningitis. Eur J Clin Microbiol Infect Dis. 2017;36:1367–72.
Anjum SH, Bennett JE, Dean O, Marr KA, Hammoud DA, Williamson PR. Neuroimaging of cryptococcal meningitis in patients without human immunodeficiency virus: data from a multi-center cohort study. J Fungi. 2023;9:594.
Tu J, Zhang S, Liu Q, Lin Y. Cerebral infarction in HIV-negative patients with cryptococcal meningitis: its predictors and impact on outcomes. BMC Infect Dis. 2022. https://doi.org/10.1186/s12879-022-07827-z.
Tater P, Pandey S. Post-stroke movement disorders: clinical spectrum, pathogenesis, and management. Neurol India. 2021;69:272–83.
Chen X, Chen J, Song Y, Su X. Vagal α7nAChR signaling regulates α7nAChR+Sca1+ cells during lung injury repair. Stem Cell Res Ther. 2020;11:375.
Shao H, Li S. A new perspective on HIV: effects of HIV on brain-heart axis. Front Cardiovasc Med. 2023;10:1226782.
Tahsili-Fahadan P, Geocadin RG. Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ Res. 2017;120:559–72.
Taggart P, Critchley H, Lambiase PD. Heart-brain interactions in cardiac arrhythmia. Heart. 2011;97:698–708.
Seong J, Kang JY, Sun JS, Kim KW. Hypothalamic inflammation and obesity: a mechanistic review. Arch Pharm Res. 2019;42:383–92.
Wang Q, Liu Y, Han L, et al. Risk factors for acute stroke-associated pneumonia and prediction of neutrophil-to-lymphocyte ratios. Am J Emerg Med. 2021;41:55–9.
Patel KP, Katsurada K, Zheng H. Cardiorenal syndrome: the role of neural connections between the heart and the kidneys. Circ Res. 2022;130:1601–17.
Wang H, Deng QW, Peng AN, et al. beta-arrestin2 functions as a key regulator in the sympathetic-triggered immunodepression after stroke. J Neuroinflamm. 2018;15:102.
Levinthal DJ, Strick PL. Multiple areas of the cerebral cortex influence the stomach. Proc Natl Acad Sci U S A. 2020;117:13078–83.
Dienel GA. Brain glucose metabolism: integration of energetics with function. Physiol Rev. 2019;99:949–1045.
Dechandt CRP, Ferrari GD, Dos Santos JR, et al. Energy metabolism and redox state in brains of wistar audiogenic rats, a genetic model of epilepsy. Front Neurol. 2019;10:1007.
Besson M-T, Dupont P, Fridell Y-WC, Liévens J-C. Increased energy metabolism rescues glia-induced pathology in a Drosophila model of Huntington’s disease. Hum Mol Genet. 2010;19:3372–82.
Robinson PA, Bauer M, Leal MAE, et al. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1999;28:82–92.
Edate S, Albanese A. Management of electrolyte and fluid disorders after brain surgery for pituitary/suprasellar tumours. Horm Res Paediatr. 2015;83:293–301.
Nessel I, Michael-Titus AT. Lipid profiling of brain tissue and blood after traumatic brain injury. Semin Cell Dev Biol. 2021;112:145–56.
Li J, Imano H, Yamagishi K, et al. Serum albumin and risks of stroke and its subtypes - the Circulatory Risk in Communities Study (CIRCS) -. Circ J. 2021;85:385–92.
Pascoe MC, Skoog I, Blomstrand C, Linden T. Albumin and depression in elderly stroke survivors: an observational cohort study. Psychiatry Res. 2015;230:658–63.
Li S, Zheng Y, Long Q, et al. Drug–drug interactions between propofol and ART drugs: inhibiting neuronal activity by affecting glucose metabolism. CNS Neurosci Therapeut. 2023. https://doi.org/10.1111/cns.14437.
Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26:223–7.
Tauber SC, Eiffert H, Kellner S, et al. Fungal encephalitis in human autopsy cases is associated with extensive neuronal damage but only minimal repair. Neuropathol Appl Neurobiol. 2014;40:610–27.
Chang YC, Stins MF, McCaffery MJ, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun. 2004;72:4985–95.
Shi M, Li SS, Zheng C, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120:1683–93.
Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–7.
Woo YH, Martinez LR. Cryptococcus neoformans-astrocyte interactions: effect on fungal blood brain barrier disruption, brain invasion, and meningitis progression. Crit Rev Microbiol. 2021;47:206–23.
Simpson IA. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann Neurol. 1994;32:546–51.
Alves VS, Leite-Aguiar R, Silva JPD, Coutinho-Silva R, Savio LEB. Purinergic signaling in infectious diseases of the central nervous system. Brain Behav Immun. 2020;89:480–90.
Niccoli T, Cabecinha M, Tillmann A, et al. Increased glucose transport into neurons rescues abeta toxicity in Drosophila. Curr Biol. 2016;26:2291–300.
Generoso JS, Thorsdottir S, Collodel A, et al. Dysfunctional glymphatic system with disrupted aquaporin 4 expression pattern on astrocytes causes bacterial product accumulation in the CSF during pneumococcal meningitis. mBio. 2022;13: e0188622.
Rottenberg DA, Moeller JR, Strother SC, Sidtis JJ, Navia BA, Dhawan V, Ginos JZ, Price RW. The metabolic pathology of the AIDS dementia complex. Ann Neurol. 1987;22(6):700–6.
Ferrucci A, Nonnemacher MR, Cohen EA, Wigdahl B. Extracellular human immunodeficiency virus type 1 viral protein R causes reductions in astrocytic ATP and glutathione levels compromising the antioxidant reservoir. Virus Res. 2012;167:358–69.
Velasquez S, Prevedel L, Valdebenito S, et al. Circulating levels of ATP is a biomarker of HIV cognitive impairment. EBioMedicine. 2020;51: 102503.
Tsai WC, Lien CY, Lee JJ, et al. The clinical characteristics of adult cryptococcal meningitis patients who died within one year of treatment with a focus on those with early mortality. J Clin Neurosci. 2019;67:80–4.
Shahan B, Choi EY, Nieves G. Cerebrospinal fluid analysis. Am Fam Physician. 2021;103:422–8.
Parihar R, Shukla R, Baishya B, Kalita J, Haldar R, Misra UK. NMR based CSF metabolomics in tuberculous meningitis: correlation with clinical and MRI findings. Metab Brain Dis. 2022;37:773–85.
Shahan B, Choi EY, Nieves G. Cerebrospinal fluid analysis. Am Fam Physic. 2021;103:422–8.
Yang Y-C, Liu S-H, Hsu Y-H, Wu Y-L, Chu P-T, Lin P-C. Cerebrospinal fluid predictors of shunt-dependent hydrocephalus after hemorrhagic stroke: a systematic review and meta-analysis. Neurosurg Rev. 2022;45:1847–59.
Schousboe A. Content of ATP in cultivated neurons and astrocytes exposed to balanced and potassium-rich media. Neurochem. 1970;17:1501–4.
Brekke E. Glucose and intermediary metabolism and astrocyte-neuron interactions following neonatal hypoxia-ischemia in rat. Neurochem Res. 2017;42:115–32.
Zhang C, Tan Z, Tian F. Impaired consciousness and decreased glucose concentration of CSF as prognostic factors in immunocompetent patients with cryptococcal meningitis. BMC Infect Dis. 2020;20:69.
Croteau E. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp Gerontol. 2018;107:18–26.
Chen SHM, Stins MF, Huang SH, et al. Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J Med Microbiol. 2003;52:961–70.
Gerhart DZ, Broderius MA, Borson ND, Drewes LR. Neurons and microvessels express the brain glucose transporter protein GLUT3. Proc Natl Acad Sci USA. 1992;89:733–7.
Banerjee D, Bloom ALM, Panepinto JC. Opposing PKA and Hog1 signals control the post-transcriptional response to glucose availability in Cryptococcus neoformans. Mol Microbiol. 2016;102:306–20.
Zhang C, He Z, Tan Z, Tian F. The clinic-based predictive modeling for prognosis of patients with cryptococcal meningitis. BMC Infect Dis. 2023. https://doi.org/10.1186/s12879-023-08337-2.
Hegen H, Walde J, Auer M, Deisenhammer F. Cerebrospinal fluid:serum glucose ratio in the ventricular and lumbar compartments: implications for clinical practice. Eur J Neurol. 2017;25:373–9.
Tan QC, Xing XW, Zhang JT, et al. Correlation between blood glucose and cerebrospinal fluid glucose levels in patients with differences in glucose metabolism. Front Neurol. 2023. https://doi.org/10.3389/fneur.2023.1239968.
Nergiz S, Aydin Ozturk P. The prognostic nutritional index and mortality in patients with ventriculoperitoneal shunt infection. Clin Pediat. 2023. https://doi.org/10.1177/00099228231209725.
Jiao FY, Cao HC, Liu ZY, Wu S, Wong HB. The use of blood glucose/cerebrospinal fluid glucose ratio in the diagnosis of central nervous system infection in infants and children. J Singapore Paediat Soc. 1992;34:191–8.
Zheng H, Chen Q, Xie Z, et al. A retrospective research of HIV-negative cryptococcal meningoencephalitis patients with acute/subacute onset. Eur J Clin Microbiol Infect Dis. 2016;35:299–303.
Abassi M, Bangdiwala AS, Nuwagira E, et al. Cerebrospinal fluid lactate as a prognostic marker of disease severity and mortality in cryptococcal meningitis. Clin Infect Dis. 2021;73:e3077–82.
Huang Y, Zou J, Zhang K, Li H, Hu D, Liao W, Zhang L, Pan W, et al. Prediction of hospital discharge outcome from changes in cerebrospinal fluid/serum albumin quotient and cerebrospinal fluid lactate dehydrogenase in patients with cryptococcal meningitis. Future Microbiol. 2022;17:223–33.
Suzuki A. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–23.
Mächler P. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 2016;23:94–102.
Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–7.
Gao C, Wang C, Liu B, et al. Intermittent hypoxia preconditioning-induced epileptic tolerance by upregulation of monocarboxylate transporter 4 expression in rat hippocampal astrocytes. Neurochem Res. 2014;39:2160–9.
Li B, Freeman RD. Neurometabolic coupling between neural activity, glucose, and lactate in activated visual cortex. J Neurochem. 2015;135:742–54.
Gao C. Intermittent hypoxia preconditioning-induced epileptic tolerance by upregulation of monocarboxylate transporter 4 expression in rat hippocampal astrocytes. Neurochem Res. 2014;39:2160–9.
Brekke E. Glucose and intermediary metabolism and astrocyte-neuron interactions following neonatal hypoxia-ischemia in rat. Neurochem Res. 2017;42:115–32.
Nussinovitch M, Finkelstein Y, Elishkevitz KP, et al. Cerebrospinal fluid lactate dehydrogenase isoenzymes in children with bacterial and aseptic meningitis. Transl Res. 2009;154:214–8.
Kellogg RT, Park MS, Snyder MH, et al. Establishment of age- and sex-specific reference cerebral ventricle volumes. World Neurosurgery. 2023;175:e976–83.
Mangoni AA, Zinellu A. A systematic review and meta-analysis of serum concentrations of ischaemia-modified albumin in acute ischaemic stroke, intracerebral haemorrhage, and subarachnoid haemorrhage. Biomolecules. 2022;12:653.
Sekhon MS, McLean N, Henderson WR, Chittock DR, Griesdale DEG. Association of hemoglobin concentration and mortality in critically ill patients with severe traumatic brain injury. Crit Care Med. 2012;16:R128.
Bahr NC, Rolfes MA, Musubire A, et al. Standardized electrolyte supplementation and fluid management improves survival during amphotericin therapy for cryptococcal meningitis in resource-limited settings. Open Forum Infect Dis. 2014. https://doi.org/10.1093/ofid/ofu070.
Liu J, Liu J, Su X, et al. Amphotericin B plus fluorocytosine combined with voriconazole for the treatment of non-HIV and non-transplant-associated cryptococcal meningitis: a retrospective study. BMC Neurol. 2022. https://doi.org/10.1186/s12883-022-02803-1.
Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–82.
Lopez-Gambero AJ, Martinez F, Salazar K, Cifuentes M, Nualart F. Brain glucose-sensing mechanism and energy homeostasis. Mol Neurobiol. 2019;56:769–96.
Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9:463–73.
Simon Á, Oláh J, Komlósi I, et al. Changes in expression of neuropeptides and their receptors in the hypothalamus and gastrointestinal tract of calorie restricted hens. Acta Biol Hung. 2017;68:237–47.
Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–34.
Akmayev IG. Popov AP Morphological aspects of the hypothalamic-hypophyseal system. VII. The tanycytes_ Their relation to the hypophyseal adrenocorticotrophic function. An ultrastructural study. Cell Tissue Res. 1977;180:263–82.
Ebling FJP, Lewis JE. Tanycytes and hypothalamic control of energy metabolism. Glia. 2018;66:1176–84.
Imbernon M, Saponaro C, Helms HCC, et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metab. 2022;34:1054-1063.e1057.
Acknowledgements
Medical Writing and Editorial Assistance.
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding
The journal's Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Jianglong Qin and Lanwei Nong: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing, and supervision. Qingdong Zhu and Zhizhong Huang: conceptualization, formal analysis, investigation, methodology, and writing. Fengyao Wu: conceptualization, statistical analysis, and writing. Sijun Li: conceptualization, writing and editing, and supervision. All authors agreed to the study design and have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Jianglong Qin, Lanwei Nong, Qingdong Zhu, Zhizhong Huang, Fengyao Wu and Sijun Li declare that they have no competing interests.
Ethical Approval
This study was approved by the Fourth People’s Hospital of Nanning and registered under project number [2023] 69. The Ethics Committee of the Fourth People’s Hospital of Nanning waived patient informed consent because we used anonymous patient electronic medical records in this study. The study was in accordance with the Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Qin, J., Nong, L., Zhu, Q. et al. A Retrospective Analysis of Central and Peripheral Metabolic Characteristics in Patients with Cryptococcal Meningitis. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00610-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00610-z