Abstract
Introduction
Clopidogrel resistance causes recurrent stroke. However, outcomes of modified antiplatelet medications to prevent recurrent ischemic stroke are not well known.
Methods
Patients who received clopidogrel with and without modification as initial treatment for stroke were recruited and compared. The primary outcome was ischemic stroke and myocardial infarction at the 1-year follow-up. The secondary outcome was bleeding complications.
Results
Overall, 206 patients treated with clopidogrel were enrolled and were divided into the modification (n = 39) and no modification (n = 167) groups. There was a significant difference in the incidence of severe cerebral arterial stenosis between the two groups (modification group, 16/39, 41.03%; no modification group, 36/167, 21.56%, P = 0.012) at baseline. The loss to follow-up rate was 12.14% (25/206). After adjustment for severe cerebral artery stenosis, antiplatelet modification based on the platelet reactivity unit (PRU) value significantly improved in the per protocol set (odds ratio 0.142, 95% confidential interval 0.022–0.898, P = 0.038). The area under the curve of the different PRU cutoff values were 0.630, 0.605, and 0.591 (P = 0.016, 0.051, and 0.092) for PRU 190, 208, and 235, respectively.
Conclusion
Verifynow P2Y12 PRU-guided modification of clopidogrel for ischemic stroke significantly improved or prevented recurrence at the 1-year follow-up. Our findings suggest that clopidogrel therapy based on the PRU cutoff value of 190 should be considered to improve outcomes.
Trial Registration
ClinicalTrials.gov NCT02618265 (December 1, 2015).
Similar content being viewed by others
Clopidogrel, which is frequently used to prevent ischemic stroke, may cause treatment resistance and lead to recurrence of stroke. |
Overall, 206 participants who received clopidogrel treatment were enrolled in the study. The participants were further divided into the modification (39 patients) and no modification (167 patients) groups. |
Modification based on the platelet reactivity unit (PRU) cutoff value significantly improved the outcomes of the per protocol set population after adjusting for severe cerebral artery stenosis. |
Clopidogrel therapy with a PRU cutoff value of 190 may be considered to improve outcomes. |
Introduction
Ischemic stroke is one of the most common causes of death and long-term disability worldwide [1]. According to the China National Stroke Registry, recurrent stroke accounts for nearly 17.7% of cases of ischemic stroke [2, 3]. Antiplatelet therapy is recommended by many guidelines to reduce the risk of recurrent ischemic stroke [4, 5], with clopidogrel being one of the most frequently prescribed medications to prevent acute ischemic stroke in high-risk patients [5,6,7,8]. However, there are interindividual variations in terms of platelet reactivity to clopidogrel therapy. According to the different test methods and reports, the prevalence of clopidogrel resistance (CR) ranges from 8% to 50% [9,10,11]. Studies have suggested that CR is a strong predictor of stroke and adverse vascular events [12,13,14]. It was demonstrated that platelet inhibition function test-guided modifications of antiplatelet medications were associated with significantly higher rates of adverse clinical outcomes [15], while another study indicated that P2Y12 platelet function test-guided intensification of antiplatelet therapy to achieve short-term inhibition of at least 20% did not result in a higher incidence of ischemic or hemorrhagic events [16].
Higashiguchi et al. indicated that personalized dual antiplatelet therapy based on a platelet reaction unit (PRU) cutoff value of 240 significantly reduced thromboembolic complications without increasing hemorrhagic complications [17] during the endovascular period. However, limited information exists regarding the efficacy of antiplatelet modification based on the Verifynow P2Y12 platelet function test [18]. Therefore, the aim of our study was to investigate the clinical outcomes associated with platelet function test-guided clopidogrel modification in patients with ischemic stroke. The hypothesis was that treatment with modified antiplatelets based on platelet function testing would decrease the incidence of recurrent stroke and bleeding.
Methods
Study Design and Participants
The prospective cohort study enrolled consecutive patients with acute ischemic stroke and transient ischemic attack (TIA) from the neurology departments of three hospitals. The diagnosis of acute ischemic stroke or TIA was established according to the criteria of the American Stroke Association (ASA) [19]. Consecutive participants were sequentially enrolled at Peking University Third Hospital from August 22, 2016 to June 6, 2017, Peking University Shougang Hospital from June 22, 2017 to March 31, 2018, and Huilongguan Branch of Beijing Jishuitan Hospital from April 9, 2018 to July 16, 2019.
The inclusion criteria were as follows:
-
1.
Age > 18 years
-
2.
Cerebral infarction with evidence on computed tomography (CT) or magnetic resonance imaging (MRI) without coma
-
3.
National Institutes of Health Stroke Scale (NIHSS) score 0–25
-
4.
TIA
-
5.
Available detailed medical history
-
6.
CT angiography (CTA) or MR angiography (MRA) of the brain and carotid arteries or color duplex ultrasound investigation of the carotid arteries
-
7.
At least 7 days of clopidogrel therapy (75 mg daily) alone or combined with aspirin (100 mg daily) prior to the Verifynow P2Y12 test
Routine electrocardiogram (ECG), 24-h Holter ECG, and ultrasound cardiogram were performed to reveal possible cardio-embolic stroke. All patients who were admitted to the ward received therapeutic treatments according to the 2013 American Heart Association (AHA)/ASA guidelines for acute ischemic stroke and 2014 guidelines for prevention of recurrent ischemic stroke.
During the screening period, a series of investigations, including blood sampling, was performed to classify stroke as one of five subtypes according to the Trial of Org 10172 in Acute Stroke Treatment criteria. Only large vessel atherosclerotic and small vessel disease subtypes were included. Adherence was determined by interviewing the patients and caregivers.
The exclusion criteria were as follows:
-
1.
Dementia
-
2.
Evidence of hemorrhage on CT or MRI
-
3.
Hematological disorders
-
3.1
Platelet count < 100 × 109/L or > 450 × < 109/L and a hemoglobin level < 8 g/dL
-
3.2
Any history of myeloproliferative disorders, heparin-induced thrombocytopenia, or any other platelet function disorders
-
3.3
Malignant paraproteinemias or a family or personal history of bleeding disorders
-
3.1
-
4.
Any major surgical procedure within 7 days prior to enrollment
-
5.
Any clinically relevant arrhythmia on admission, including atrial fibrillation
-
6.
Any major concurrent illness, including severe cardiovascular disease, liver or renal failure, and malignancies
-
7.
Fever, hypoxia, alterations in consciousness, or any relevant hemodynamic compromise on admission
-
8.
Nonadherence to antiplatelet therapy
-
9.
Any allergy to aspirin or clopidogrel
-
10.
Asthma
-
11.
Concurrently treated with glycoprotein IIb/IIIa inhibitors, ticlopidine, dipyridamole, or other additional antiplatelets and other nonsteroidal antiinflammatory drugs at the time of the index stroke
-
12.
Administration of heparin or low molecular weight heparin or other anticoagulants within 24 h before enrollment in the study
-
13.
Any patients deemed as not suitable for enrollment
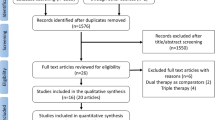
The administration and modification of specific antiplatelet medications were recorded. The study was registered at ClinicalTrials.gov (NCT02618265). The study protocol was approved by the Ethics Committee of Peking University Third Hospital (2013-144) and subsites (The ethics committee approval number of Peking University Shougang Hospital is IRBK-2017-033-01. The ethics approval of Peking University Third Hospital is accepted in Beijing Jishuitan Hospital Huilongguan Branch). Participants gave written informed consent for participation. The study was conducted in accordance with the Declaration of Helsinki. Figure 1 shows the flowchart of inclusion and management.
Sample Size Estimation
We estimated that a sample size of 200 patients would provide 80% (1 − β) power at a two-tailed 5% (type I error rate, α) significance level to detect a difference between the modification and no modification groups, similar to that in the literature.
Based on a cohort study of patients with symptomatic atherothrombotic intracranial stenosis, the incidence of recurrent stroke was 25% [20, 21], which was adopted in the study. The contemporary treatment strategies included antiplatelets, statins, stent implantation, and/or internal carotid endarterectomy for prevention of recurrent ischemic stroke. The estimation assumed an endpoint rate of 9% [22] of the study population (treatment group, 9%; natural history group, 25%; sampling ratio, 1:1; superiority margin, 0.08). The sample size was estimated as 56 (each group, n = 28).
In a real-world study, all patients received clopidogrel. To test the difference between the modification and no modification groups, we speculated a 1:1 ratio since the maximum variability for CR was 50%. According to the literature, the incidences of ischemic stroke with dual and mono antiplatelet therapies were 9.1% and 27.9% [22], respectively; hence, the sample size was calculated to be 100 (superiority margin, 0.10; each group, n = 50).
A recently published study regarding scheduled neurointervention for unruptured intracranial aneurysms reported that the incidence of thromboembolic complications in the tailored and nontailored groups was 6.6% and 16% [17], respectively; according to the outcome rate of this study, the sample size was calculated to be 296 (superiority margin, 0.05; each group, n = 148).
The estimation was entered into an online sample size calculator (http://www.powerandsamplesize.com/), and the relevant parameters were set. Considering a shedding rate of less than 20%, the trial was designed to enroll at least 120 patients; 356 patients at most should be included. As a result of the disparity in the diseases from the collected studies, we selected 200 patients as the sample size.
Platelet Reactivity Assessment
The Verifynow P2Y12 assay, which is based on optical turbidimetry, is mostly similar to the gold standard, which is light transmittance aggregometry; however, the advantage of the P2Y12 assay is that it can be performed at the bedside. Adenosine diphosphate (ADP) was used as a platelet activator to detect platelet aggregation after administering a P2Y12 receptor inhibitor. Clopidogrel irreversibly binds to the P2Y12-coupled purinergic receptor, inhibiting ADP-mediated platelet activation and aggregation. ADP was used to maximally activate the platelets by binding to the P2Y1 and P2Y12 platelet receptors, while prostaglandin E1 (PGE1) was used to suppress ADP-induced P2Y1-mediated increase in intracellular calcium levels. Based on these mechanisms, the Verifynow P2Y12 assay (ADP PGE1 method) could determine maximal P2Y12 inhibition.
The Verifynow P2Y12 PRU was assessed after a 7-day course of antiplatelet therapy using clopidogrel according to the manufacturer’s protocol (Accriva, San Diego, CA, USA). Electronic and liquid quality control was implemented before each test to guarantee the comparability of the measurements among each site. The classical PRU standard of CR, wherein PRU exceeds 208, is based on the cardiovascular clinical outcomes of the GRAVITAS and ADAPT-DES research [23, 24]; a lower PRU indicates greater antiplatelet aggregation.
CR Standard and Medicine Modification Reference Consideration
To our knowledge, as a global platelet function test for clopidogrel, the PRU is correlated with the CYP2C19 loss-of-function genotype, which results in high PRU values [25]. East Asians carry the CYP2C19 polymorphism *2 and/or *3 [26], which may possibly result in resistance to treatment of our research population. However, we could not exclude ultrarapid alleles that contribute to and increase bleeding tendency or other gene polymorphisms [27, 28]. The risk prediction model S2TOP-BLEED scale [29] has defined that being Asian is a potential risk factor for hemorrhage. Owing to that, PRU perhaps will be not steady during the long-term ischemic stroke secondary prevention period of clopidogrel therapy with several uncontrollable factors, such as blood glucose level, proton pump inhibitors (PPI), and calcium channel blockers (CCB) or other traditional Chinese medicine which will influence antiplatelet effects. Thus, it is necessary to establish a slightly varied PRU to assess effective platelet inhibition and avoid heterogeneous platelet responses across individuals. The standard PRU 208 was derived from clinical research of coronary heart disease; however, the physiological pathology in cerebral and coronary vessels is different. According to our hypothesis, the PRU cutoff value for antiplatelet modification should be slightly lower than that of the standard value. Hence, a PRU cutoff value of 190 was deemed appropriate for antiplatelet modification to avoid intracranial hemorrhage and bleeding or unnoticed CR. As the hypothesis was defined by clinically experienced neurologists, the relatively lower PRU standard is potentially reasonable.
Standard Operating Procedure of Antiplatelet Modification
All patients received standard doses of either combined aspirin 100 mg and clopidogrel 75 mg daily or clopidogrel 75 mg alone once a day for at least 7 days prior to undergoing the Verifynow P2Y12 test. Antiplatelet treatment lasted for 7 days, and all participants underwent the P2Y12 platelet function test to identify patients with CR or treatment responders and determine whether they would need long-term antiplatelet modification for the prevention of recurrent ischemic stroke. For patients with TIA and mild artery stenosis, antiplatelet therapy without modification was administered, although the PRU cutoff value was set at 190. For patients with moderate stenosis and a PRU greater than 190, antiplatelet modification was considered. If artery stenosis was severe but without cerebral microbleeding, combined clopidogrel and aspirin therapy was continued for at least 3 months. For severe artery stenosis with cerebral microbleeding, combined clopidogrel and aspirin therapy was shifted to either clopidogrel or aspirin to avoid intracranial hemorrhage and bleeding. Furthermore, other factors that may influence the results, such as blood glucose medications and PPI, were administered prior to the study to decrease their influence on platelet inhibition.
Medicine Adherence
Adherence to medication was defined as the extent to which participants continuously took all medications as prescribed at hospital discharge to prevent a recurrence of ischemic stroke, except if their health care provider instructed them to discontinue a medication [30]. For all participants, the follow-up assessment included adherence to treatment. For participants whose medical records could be obtained, we calculated the prescribed dosage and frequency of drugs to determine medication adherence. For patients who resided outside the region of the hospital after discharge, we determined medication adherence at follow-up. Not adhering to medications for 2 weeks was defined as nonadherence.
Assessment of Outcomes
Stroke, including acute ischemic stroke, TIA, intracranial hemorrhage, and myocardial infarction, were defined as the primary outcome. Bleeding, including intracranial hemorrhage and intraocular, digestive tract, and skin bleeding, was defined as the secondary outcome. Outcomes were determined through telephone follow-ups by researchers and from medical records.
Data Collection
The following variables were collected and analyzed: patient demographics, including age and gender, and clinical characteristics, including medical history such as hypertension, diabetes mellitus, previous stroke, coronary heart disease, and smoking. Data from MRI, MRA, CTA, and/or digital subtraction angiography for evaluating intracranial and extracranial artery stenosis or small cerebral vessel disease were obtained. Cerebral artery stenosis on angiography was categorized as mild (< 30%), moderate (30–50%), or severe (> 50%). Concomitant medications, including antihypertensives, statins, and PPI, were recorded. Recurrent ischemic stroke, acute myocardial infarction, and bleeding were recorded on follow-up.
Statistical Analysis
We analyzed the demographic and clinical variables using descriptive statistics. The normality of distribution of PRU was exhibited by the Q–Q plot. Continuous variables were presented as mean and standard deviation. Between-group comparisons of continuous variables were performed using the independent sample t test. Non-normally distributed data were analyzed by the Wilcoxon rank-sum test. Categorical variables were presented as frequencies and analyzed by the chi-square test. The baseline characteristics of all intent-to-treat (ITT) [31] participants were compared between the modification and no modification groups. The per protocol set (PPS) population was defined as all participants who underwent follow-up assessments. We used the χ2 test and survival analysis to convey the difference in the primary outcome between the modification and no modification groups for estimating the risk ratio (RR), Kaplan–Meier plots, and log-rank test P value. According to the principle of instrumental variable analysis [32, 33], binary logistic regression analysis was performed with the modified antiplatelet as the independent variable and outcome as the dependent variable. Statistically and clinically significant severe artery stenosis was set as the covariate variable. The odds ratios (ORs) between the modification and no modification groups were expressed with 95% confidence intervals (CIs) and P value. To investigate the best PRU cutoff value for clopidogrel modification, we calculated the area under the curve (AUC) of the receiver operating characteristic (ROC). PRU values were entered into the calculation process. The Youden index was calculated using the equation “specificity + sensitivity − 1,” with the maximum corresponding PRU value as the best cutoff value. Statistical significance was defined as a two-tailed P value less than 0.05. Statistical analyses were carried out by SPSS statistical software version 20.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline Demographics and Clinical Characteristics
Overall, 206 participants were enrolled in the study; 200 patients had acute ischemic stroke and 6 patients had TIA. The treatment modification details are listed in Table 1. The demographics and clinical characteristics among the three subsites are listed in Table 2. The comparison between the antiplatelet modification group and no modification group are listed in Table 3. There were no significant differences between the antiplatelet modification and no modification groups except for the proportion of patients with severe cerebral arterial stenosis, which was evident in both the ITT and PPS sets. Initial therapies were monotherapy (34.47% clopidogrel) or dual antiplatelet (65.53% clopidogrel and aspirin) therapy. All participants received statin treatment. The normality of distribution of the PRU is shown in Fig. 2 including the enrolled population and modification group. Based on the PRU cutoff values of 190, 208, and 235, the overall prevalence of CR was 39.81% (82/206), 28.64% (59/206), and 17.48% (36/206), respectively [25].
There was no statistically significant difference in the use of β-blockers, CCB, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers between the modification and no modification groups. Table 4 shows the differences between patients with CR and clopidogrel responders according to the PRU cutoff value of 190 (ITT and PPS analysis). The age (ITT: 64.58 ± 11.09, PPS: 63.97 ± 10.49) and in-hospital NIHSS (ITT: 2.84 ± 2.59, PPS: 3.01 ± 2.67) of the patients with CR were higher than in clopidogrel responders (ITT: 59.04 ± 11.27, PPS: 58.95 ± 10.71, ITT set P = 0.001*, PPS set P = 0.003*; ITT: 2.07 ± 2.15, PPS: 2.04 ± 2.20, ITT set P = 0.028*, PPS set P = 0.013*, respectively). There were less smokers among patients with CR (ITT: 32.93%, PPS: 54.84%) than in clopidogrel responders (ITT: 35.82%, PPS: 56.14%; ITT set P = 0.002*, PPS set P = 0.008*).
Primary Outcome of the PPS Population
The dropout rate was 12.14% (25/206). The outcome rates of the modification and no modification groups were 13.89% (5/36) and 8.28% (12/145), respectively. The RR of the modification was 1.149 (95% CI 0.838–1.575, P = 0.475), which indicated that there was no significant difference between the modification and no modification groups. Additionally, Kaplan–Meier analysis showed that there was no significant difference between the modification and no modification groups (log-rank test, P = 0.718, Fig. 3). However, there was a significant difference in the incidence of severe cerebral arterial stenosis between the modification and no modification groups (ITT: 41.03% vs. 21.56%, P = 0.012*; PPS: 41.67% vs. 24.83%, P < 0.001*). Of the 181 participants who completed the 1-year follow-up, 38.89% (14/36) and 6.21% (9/145) in the modification and no modification groups had severe stenosis and had not undergone implantation and/or carotid endarterectomy, respectively. Table 5 indicates that in the two steps of logistic regression, there was no statistically significant difference between modification and no modification group as for other statistics. After adopting severe cerebral arterial stenosis as a covariate variable, logistic regression analysis showed that there was a significant difference in the primary outcome between the modification and no modification groups (OR 0.142, 95% CI 0.022–0.898, P = 0.038). The results indicated that according to the PRU cutoff value, antiplatelet modification decreased the incidence of recurrent stroke.
Secondary Outcomes in the Modification and No Modification Groups
Major intracranial hemorrhage was not observed in both groups. Meanwhile, petechiae were observed in the no modification group, which had a PRU value of 175. There was no significant difference between the modification and no modification groups (P = 0.628, Table 3).
Sensitivity Analysis of the PRU Cutoff Value for Antiplatelet Modification
The investigated diagnostic indexes included the sensitivity, specificity, and AUC of the different PRU cutoff values. All PPS data were included in the ROC analysis. When PRU and modification were set as the independent and dependent variables in the ROC analysis, respectively, the AUC was 0.623, P = 0.024 (95% CI 0.511–0.735). The Youden index ranged from − 0.080 to 0.245. With the best value of 0.245, the corresponding PRU cutoff value was 189.5. Figure 4 shows the ROC curve of the different cutoff values. The AUCs of the PRU cutoff values for 190, 235, and 208 were 0.630 (P = 0.016, 95% CI 0.526–0.733), 0.591 (P = 0.092, 95% CI 0.480–0.701), and 0.605 (P = 0.051, 95% CI 0.497–0.713), and the sensitivity and specificity were 58.3%, 67.6%, and 30.6%, and 87.6%, 44.4%, and 76.6%, respectively. After removing outliers (PRU 5, 9, and 47), the AUC was 0.66, P = 0.004 (95% CI 0.553–0.767), and the Youden index ranged from − 0.026 to 0.280. With the best value of 0.280, the corresponding PRU value was also 189.5. The AUCs of 190, 235, and 208 were 0.647 (P = 0.008, 95% CI 0.508–0.728), 0.6 (P = 0.071, 95% CI 0.487–0.713), and 0.618 (P = 0.032, 95% CI 0.508–0.728), and the sensitivity and specificity were 61.8% and 67.6%, 32.4%, and 87.6%, and 41.7% and 76.6%, respectively.
Discussion
Our results showed that antiplatelet modification based on the P2Y12 PRU test decreases the incidence of recurrent ischemic stroke without increasing the risk of bleeding after adjusting for severe intracranial and extracranial artery stenosis. This suggests that long-term prevention of stroke recurrence may be achieved by selecting the appropriate antiplatelet medications. The outcome rate was not significantly different between the modification and no modification groups, indicating that the insufficiency of platelet inhibition induced recurrent ischemic stroke could be offset by antiplatelet modification. CR was neutralized, and the outcome rate of patients with CR was similar to that of clopidogrel responders. Moreover, after adjustment for severe artery stenosis, antiplatelet modification was more effective than no modification, which indicated that prophase modification was a more selective preference “test to therapy” strategy for better prognosis.
Aspirin and clopidogrel are prescribed for both acute stroke and prevention of recurrence. Low-dose aspirin is effective for reducing ischemic events above a certain risk threshold [34]. The Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events study reported that clopidogrel decreased the relative risk for ischemic stroke versus aspirin in patients with ischemic stroke [35]. The CARESS study reported that dual therapy with clopidogrel and aspirin was more effective than aspirin alone for reducing asymptomatic embolization of carotid stenosis [36, 37]. Nevertheless, in the Management and Avoidance trial, the combination of clopidogrel plus aspirin was not significantly more effective than aspirin alone in reducing the incidence of myocardial infarction, stroke, or death from cardiovascular causes [38]. The results are similar in patients with a lacunar stroke in the prevention of small subcortical strokes trial [39]. Thus, the combination of aspirin and clopidogrel for 21 days and then monotherapy with either drug has shown that dual antiplatelet therapy is more effective than mono aspirin treatment for decreasing recurrent ischemic stroke without increasing hemorrhage risk [6]. Combined aspirin and clopidogrel for 90 days showed that dual antiplatelet therapy decreased the incidence of recurrent ischemic stroke but increased that of hemorrhage [40]. The inconsistent results of both monotherapy and dual antiplatelet therapy could be partially attributed to the variability of platelet reactivity. Among patients with ischemic stroke or TIA treated with clopidogrel, carriers of CYP2C19 loss-of-function alleles are at greater risk of stroke and composite vascular events than noncarriers [41].
Clopidogrel is a prodrug activated by cytochrome P450. The active metabolite then binds to the P2Y12 receptor, inhibiting ADP receptor-mediated activation. As the global platelet function test for clopidogrel, a relative high PRU value is correlated with CYP2C19 loss-of-function genotype [42]. The mechanisms associated with inhibition of platelet function are complex and multifactorial, which include other genetic factors, poor compliance, dose insufficiency, drug–drug interaction, obesity, smoking, diabetes, and renal failure. To accurately reflect the degree of platelet inhibition brought about by antiplatelet modification, blood pressure, glucose level, and adherence were assessed before patients underwent the Verifynow P2Y12 test.
Platelet function testing is not recommended in the current guidelines for the management of ischemic stroke or TIA [5], and it is only recommended for high-risk patients with cardiovascular diseases. Several studies have demonstrated that high PRU values could predict thromboembolic events even in the periprocedural stage. Despite sufficient intake of dual antiplatelet therapy [43,44,45], there is a significantly high proportion of patients with CR who have high PRU values. Meanwhile, it was reported that prophylactic clopidogrel therapy may induce a potential increase in hemorrhagic events in patients with low PRU values [46]. Depta et al. implied that increasing the dose of antiplatelets would increase the risk of adverse events [15]. There are few published studies regarding the incidence of periprocedural thromboembolic events after changing regimen of dual antiplatelet therapy tends to decrease in patients with CR [18]. Previous research showed that 69% of patients with CR were treated with increasing doses of up to 150 mg of antiplatelets daily [47], which may reduce CR prevalence by 86.6% while having nonsignificant hemorrhage (major bleeding events) as demonstrated by clopidogrel responders and patients with CR. Our results are comparable to that of a study on antiplatelet therapy modification during the endovascular perioperative period, which showed that modification significantly reduces the frequency of thromboembolic complications without increasing hemorrhagic complications at 3 months follow-up [17]. A PRU cutoff value of 190 to guide antiplatelet modification is a feasible strategy to reduce adverse clinical outcomes, with petechiae as the only bleeding outcome in our study. Although the cutoff values were derived from different sources, 190 was the most sensitive for antiplatelet modification while demonstrating favorable outcomes.
Previous research showed that long-term (more than 90 days) high-dose antiplatelet therapy did not decrease recurrent stroke but increased bleeding [15]; hence, other drugs, such as cilostazol or ticagrelor, should be selected to decrease the risk of adverse events [48, 49].
To identify the most potent modifiable antiplatelet drug to prevent recurrent ischemic stroke while maintaining a low risk of bleeding, a prospective cohort study should be conducted. The strengths of our study include the standardized data collection procedures, detailed information on possible confounding factors, other treatments, and the use of the validated clinical screening instrument Verifynow P2Y12 platelet function test. The limitation of this study is its nonrandomized design, lack of causality, and small sample size. Although a PRU value of less than 95, which has a high risk of bleeding, is uncommon, bedside testing and antiplatelet modification are useful tools to prevent recurrent stroke.
Conclusion
Verifynow P2Y12 PRU-guided modification of clopidogrel significantly reduced the incidence of recurrent ischemic stroke.
References
Wang Y, Cui L, Ji X, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. 2011;6(4):355–61.
Wang Y, Xu J, Zhao X, et al. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke. 2013;44(5):1232–7.
Wang Z, Li J, Wang C, et al. Gender differences in 1-year clinical characteristics and outcomes after stroke: results from the China National Stroke Registry. PLoS ONE. 2013;8(2):e56459.
Wang YJ, Zhang SM, Zhang L, et al. Chinese guidelines for the secondary prevention of ischemic stroke and transient ischemic attack 2010. CNS Neurosci Ther. 2012;18(2):93–101.
Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236
Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11–9.
Kim JT, Park MS, Choi KH, et al. Comparative effectiveness of aspirin and clopidogrel versus aspirin in acute minor stroke or transient ischemic attack. Stroke. 2019;50:101–109.
Zhang Q, Wang C, Zheng M, et al. Aspirin plus clopidogrel as secondary prevention after stroke or transient ischemic attack: a systematic review and meta-analysis. Cerebrovasc Dis. 2015;39(1):13–22.
Fukuoka T, Furuya D, Takeda H, et al. Evaluation of clopidogrel resistance in ischemic stroke patients. Intern Med. 2011;50(1):31–5.
Fong J, Cheng-Ching E, Hussain MS, Katzan I, Gupta R. Predictors of biochemical aspirin and clopidogrel resistance in patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2011;20(3):227–30.
Gurbel PA, Antonino MJ, Tantry US. Recent developments in clopidogrel pharmacology and their relation to clinical outcomes. Expert Opin Drug Metab Toxicol. 2009;5(8):989–1004.
Alakbarzade V, Huang X, Ster IC, McEntagart M, Pereira AC. High on-clopidogrel platelet reactivity in ischaemic stroke or transient ischaemic attack: systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020;29(7):104877.
Yi X, Lin J, Zhou Q, Wu L, Cheng W, Wang C. Clopidogrel resistance increases rate of recurrent stroke and other vascular events in Chinese population. J Stroke Cerebrovasc Dis. 2016;25(5):1222–8.
Yi X, Lin J, Wang Y, Zhou J, Zhou Q, Wang C. Response to clopidogrel is associated with early neurological deterioration after acute ischemic stroke. Oncotarget. 2018;9(28):19900–10.
Depta JP, Fowler J, Novak E, et al. Clinical outcomes using a platelet function-guided approach for secondary prevention in patients with ischemic stroke or transient ischemic attack. Stroke. 2012;43(9):2376–81.
Nordeen JD, Patel AV, Darracott RM, et al. Clopidogrel resistance by P2Y12 platelet function testing in patients undergoing neuroendovascular procedures: incidence of ischemic and hemorrhagic complications. J Vasc Interv Neurol. 2013;6(1):26–34.
Higashiguchi S, Sadato A, Nakahara I, et al. Reduction of thromboembolic complications during the endovascular treatment of unruptured aneurysms by employing a tailored dual antiplatelet regimen using aspirin and prasugrel. J Neurointerv Surg. 2021;13(11):1044–8.
Kim HJ, Oh JS, Park SQ, Yoon SM, Ahn HS, Kim BT. The efficacy of P2Y12 reactive unit to predict the periprocedural thromboembolic and hemorrhagic complications according to clopidogrel responsiveness and safety of modification of dual antiplatelet therapy: a meta-analysis. J Korean Neurosurg Soc. 2020;63(5):539–49.
Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276–93.
Mazighi M, Tanasescu R, Ducrocq X, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006;66(8):1187–91.
Kern R, Steinke W, Daffertshofer M, Prager R, Hennerici M. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. 2005;65(6):859–64.
Zuo FT, Liu H, Wu HJ, Su N, Liu JQ, Dong AQ. The effectiveness and safety of dual antiplatelet therapy in ischemic cerebrovascular disease with intracranial and extracranial arteriostenosis in Chinese patients: a randomized and controlled trail. Med (Baltim). 2017;96(1):e5497.
Price MJ, Angiolillo DJ, Teirstein PS, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the gauging responsiveness with a VerifyNow P2Y12 assay: impact on thrombosis and safety (GRAVITAS) trial. Circulation. 2011;124(10):1132–7.
Stone GW, Witzenbichler B, Weisz G, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382(9892):614–23.
Wang Z, Xie Q, Xiang Q, Gong Y, Jiang J, Cui Y. Predictive value of methods measuring platelet activation for ischemic events in patients receiving clopidogrel: a systematic review and meta-analysis. Curr Pharm Des. 2018;24(44):5313–33.
Dahabreh IJ, Moorthy D, Lamont JL, Chen ML, Kent DM, Lau J. Testing of CYP2C19 variants and platelet reactivity for guiding antiplatelet treatment [Internet]. Rockville (MD): Agency for Healthcare Research and Quality. US; 2013 Sep. Report No.: 13-ehc117-ef.
Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316(1):70–8.
Lu SJ, Zhou XS, Zheng Q, Chen HL, Geng YL. Platelet membrane receptor P2Y12 H1/H2 polymorphism is highly associated with cerebral infarction: a case-control study. Neuropsychiatr Dis Treat. 2018;14:2225–31.
Hilkens NA, Algra A, Diener HC, et al. Predicting major bleeding in patients with noncardioembolic stroke on antiplatelets: S2TOP-BLEED. Neurology. 2017;89(9):936–43.
Serebruany V, Cherala G, Williams C, et al. Association of platelet responsiveness with clopidogrel metabolism: role of compliance in the assessment of “resistance.” Am Heart J. 2009;158(6):925–32.
Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol. 1992;21(5):837–41.
Brookhart MA, Wang PS, Solomon DH, Schneeweiss S. Instrumental variable analysis of secondary pharmacoepidemiologic data. Epidemiology. 2006;17(4):373–4.
Swanson SA. Instrumental variable analyses in pharmacoepidemiology: what target trials do we emulate? Curr Epidemiol Rep. 2017;4(4):281–7.
Antithrombotic Trialists’ Collaboration. Meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348(9038):1329–39.
Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial. Circulation. 2005;111(17):2233–40.
Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9(5):489–97.
Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–17.
Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817–25.
Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215–25.
Pan Y, Chen W, Xu Y, et al. Genetic polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Circulation. 2017;135(1):21–33.
Kang HG, Lee SJ, Heo SH, Chang DI, Kim BJ. Clopidogrel resistance in patients with stroke recurrence under single or dual antiplatelet treatment. Front Neurol. 2021;12:652416.
Jiang B, Bender MT, Westbroek EM, et al. Procedural complexity independent of P2Y12 reaction unit (PRU) values is associated with acute in situ thrombosis in pipeline flow diversion of cerebral aneurysms. Stroke Vasc Neurol. 2018;3(3):169–75.
Fifi JT, Brockington C, Narang J, et al. Clopidogrel resistance is associated with thromboembolic complications in patients undergoing neurovascular stenting. AJNR Am J Neuroradiol. 2013;34(4):716–20.
Prabhakaran S, Wells KR, Lee VH, Flaherty CA, Lopes DK. Prevalence and risk factors for aspirin and clopidogrel resistance in cerebrovascular stenting. AJNR Am J Neuroradiol. 2008;29(2):281–5.
Wong P, Tesoro E, Aletich V, Alaraj A. Accumetrics-based clopidogrel dosing in endovascular neurosurgery. Neurol Res. 2015;37(11):998–1005.
Neubauer H, Kaiser AF, Endres HG, et al. Tailored antiplatelet therapy can overcome clopidogrel and aspirin resistance–the BOchum CLopidogrel and Aspirin Plan (BOCLA-Plan) to improve antiplatelet therapy. BMC Med. 2011;9:3.
Maruyama H, Fukuoka T, Deguchi I, et al. Dual antiplatelet therapy clopidogrel with low-dose cilostazol intensifies platelet inhibition in patients with ischemic stroke. Intern Med. 2013;52(10):1043–7.
Yang Y, Chen W, Pan Y, et al. Ticagrelor is superior to clopidogrel in inhibiting platelet reactivity in patients with minor stroke or TIA. Front Neurol. 2020;11:534.
Acknowledgements
The authors would like to thank all the participants for their support.
Funding
The research received grants from Capital Health Research and Development of Major Special Project (2014-1-4092). The Rapid Service Fee funding sponsor is Peking University Third Hospital.
Editorial Assistance
Editorial assistance was received from Jeffrey K (Enago) and funded by Peking University Third Hospital.
Author Contributions
Yuanjin Zhang contributed to manuscript writing, data analysis, research design and administration, and Verifynow test investigation. Dongsheng Fan contributed to application fund, manuscript revision, research design, and administration. Others contributed to Verifynow test investigation and subsite administration.
Disclosures
Yuanjin Zhang, Dongsheng Fan, Shudong Qiao, and Hongtao Hu have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the Ethics Committee of Peking University Third Hospital (2013-144) and subsites (The ethics committee approval number of Peking University Shougang Hospital is IRBK-2017-033-01. The ethics approval of Peking University Third Hospital is accepted in Beijing Jishuitan Hospital Huilongguan Branch). Participants gave written informed consent for participation. The study was conducted in accordance with the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, Y., Fan, D., Qiao, S. et al. Verifynow P2Y12 PRU-Guided Modification of Clopidogrel for Prevention of Recurrent Ischemic Stroke: A Real-World Prospective Cohort Study. Neurol Ther 11, 1749–1766 (2022). https://doi.org/10.1007/s40120-022-00406-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00406-z