Abstract
Purpose
We aimed to assess symptoms in patients after SARS-CoV-2 infection and to identify factors predicting prolonged time to symptom-free.
Methods
COVIDOM/NAPKON-POP is a population-based prospective cohort of adults whose first on-site visits were scheduled ≥ 6 months after a positive SARS-CoV-2 PCR test. Retrospective data including self-reported symptoms and time to symptom-free were collected during the survey before a site visit. In the survival analyses, being symptom-free served as the event and time to be symptom-free as the time variable. Data were visualized with Kaplan–Meier curves, differences were tested with log-rank tests. A stratified Cox proportional hazard model was used to estimate adjusted hazard ratios (aHRs) of predictors, with aHR < 1 indicating a longer time to symptom-free.
Results
Of 1175 symptomatic participants included in the present analysis, 636 (54.1%) reported persistent symptoms after 280 days (SD 68) post infection. 25% of participants were free from symptoms after 18 days [quartiles: 14, 21]. Factors associated with prolonged time to symptom-free were age 49–59 years compared to < 49 years (aHR 0.70, 95% CI 0.56–0.87), female sex (aHR 0.78, 95% CI 0.65–0.93), lower educational level (aHR 0.77, 95% CI 0.64–0.93), living with a partner (aHR 0.81, 95% CI 0.66–0.99), low resilience (aHR 0.65, 95% CI 0.47–0.90), steroid treatment (aHR 0.22, 95% CI 0.05–0.90) and no medication (aHR 0.74, 95% CI 0.62–0.89) during acute infection.
Conclusion
In the studied population, COVID-19 symptoms had resolved in one-quarter of participants within 18 days, and in 34.5% within 28 days. Over half of the participants reported COVID-19-related symptoms 9 months after infection. Symptom persistence was predominantly determined by participant’s characteristics that are difficult to modify.
Similar content being viewed by others
Introduction
As of December 2022, severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infection has been confirmed in over 600 million people worldwide [1]. Many patients, even those with mild-to-moderate acute symptoms, continue to suffer from symptoms after acute disease [2, 3]. “Long COVID” is increasingly used as an umbrella term for signs and symptoms persisting for 4 weeks or longer after SARS-CoV-2 infection [4].
The most frequently reported persisting symptoms include fatigue, dyspnea, sleep disorders or insomnia, headache, attention disorders, anosmia and ageusia [5,6,7,8,9,10]. A systematic review of 151 studies revealed that > 50% of COVID-19 patients still had at least one symptom 12 months after a confirmed infection [11]. However, generalizability to the general population is hampered by the fact that many studies investigating persisting symptoms after SARS-CoV-2 infection were based on hospitalized patients whilst others drew upon small, selected samples, or lacked a sufficiently long follow-up period [12,13,14,15,16]. The ongoing German COVIDOM/NAPKON-POP population-based study included participants ≥ 6 months after a positive SARS-CoV-2 polymerase chain reaction (PCR) test, regardless of disease severity. Recently, some of us used the first results of this study [9] to develop a severity score to quantify the symptom load associated with post-COVID syndrome (PCS score), which is broadly synonymous with Long COVID. PCS score facilitates an objective assessment of the extent and severity of the condition in the general population. However, detailed information on the health burden of long COVID, specifically on the time to full recovery, remains scarce.
A study from the Netherlands reported a median time to complete recovery of 63 days among individuals with mild, and 232 days among individuals with moderate disease severity [17]. A large international online survey of patients with suspected and confirmed SARS-CoV-2 infection revealed that the probability of time to recovery from symptoms exceeding 35 weeks was 91.8% [18]. Most eminent risk factors for Long COVID were the presence or number of existing comorbidities [2, 17, 19], however, results on risks of individual comorbidities were inconsistent [13, 20,21,22]. Treatment during acute infection such as steroid or antibiotic medication was not indicative of a complete recovery [23]. Up to date, the time course of COVID-19 symptoms and factors associated with time to recovery are thus still incompletely understood.
Using COVIDOM/NAPKON-POP baseline data, we aimed to retrospectively assess the time course of symptom persistence after SARS-CoV-2 infection. We also investigated factors predicting prolonged time to complete recovery (i.e., to becoming symptom-free) in this multi-center population-based study covering three regions of Germany.
Methods
Study design
The National Pandemic Cohort Study Network (“Nationales Pandemie Kohorten Netz”, NAPKON) was established in Germany in 2020 to coordinate and harmonize COVID-19 research at a nation-wide level [24]. NAPKON-POP is the population-based platform that hosts the COVIDOM study aimed at investigating the long-term consequences of COVID-19. Participants in COVIDOM/NAPKON-POP were recruited at three study sites in Germany, namely Kiel, Würzburg, and the Neukölln district of Berlin, covering defined geographical regions in the vicinity.
Participants
All eligible individuals were identified through the mandatory registration of a positive SARS-CoV-2 PCR test by local health authorities. First on-site visits of prospective participants were scheduled ≥ 6 months post PCR test, regardless of their acute disease severity, following procedures detailed elsewhere [25]. Inclusion criteria of participants were: (a) positive PCR for SARS-CoV-2 ≥ 6 months before enrollment, (b) living in one of the three covered regions, (c) ≥ 18 years of age, and (d) written informed consent. Exclusion criterion was an acute SARS-CoV-2 re-infection at the time of the initial questionnaire, or at the scheduled site visit [25]. Recruitment and follow-up of the COVIDOM/NAPKON-POP cohort are still ongoing. For the present analysis, data from participants recruited between November 2020 and September 2021 were used, and only symptomatic participants were included.
Method of data collection
Retrospective data on the acute course of COVID-19, time to symptom-free and current symptoms were collected from self-filled questionnaires before the on-site visit. Later, participants were assessed at the study sites during enrollment into the prospective cohort study, collecting data on body measurement, resilience, COVID-19 treatment, comorbidities, and lifestyles by physical examination, questionnaires, and interviews [25].
Measures
Symptoms
COVID-19-related symptoms were assessed by a self-selection from 22 specific symptoms and “other symptoms” [9]. Participants were asked whether they experienced these symptoms in either the infection/acute period or at the time of the survey (“current symptoms”). Fatigue was considered present when the free-text answer to the prompting question following “other symptoms” contained “fatigue” or its synonyms. A list of all 23 symptoms is provided in Fig. 1. Presence of current symptoms was assessed by the question “Do you still have symptoms currently?”.
Time to symptom-free
Time to symptom-free was assessed using the question: “How long did it take you to become symptom-free after the occurrence of first symptoms?” Time to symptom-free was measured as the time from the first appearance of symptoms to symptom-free status in days, weeks or months, re-scaled to days (7 days per week and 30 days per month) for the purpose of the present study.
For those still experiencing symptoms at the time of the survey, time to be symptom-free was considered as censored and was calculated as the time between the appearance of the first symptoms and the survey.
Additionally, we tested for group differences up to 28 days (i.e. before becoming a Long COVID case) by manually censoring data at this time point. In detail, we set the symptom-free time to 28 days and the symptom status to “experiencing symptoms” whenever getting symptom-free took longer than 28 days.
Alcohol consumption
Alcohol consumption was categorized as abstainers, low-risk alcohol consumption, or risky alcohol consumption (i.e. ≥ 5 times per week, or consumption on one occasion ≥ 4 or ≥ 5 glasses for women and men, respectively) [26].
Body Mass Index (BMI)
BMI was calculated from the weight and height measurements taken at the study site with the formula BMI = kg/m2 and was categorized as: underweight (BMI < 18.5), normal (18.5 ≤ BMI < 25), pre-obese (25 ≤ BMI < 30), or obese (BMI ≥ 30) [27].
Resilience
Resilience was measured by the 6-item Brief Resilience Scale and was categorized as: low (1.00–2.99), normal (3.00–4.30), and high (4.31–5.00). The Brief Resilience Scale can be found in Supplementary Appendix (S Table 1).
COVID-19 treatment
COVID-19 treatment was assessed by the question: “Have you taken any medications for SARS-Cov-2 infection?” together with prompting three treatment categories of steroids, anticoagulation, and anti-infectives. In the present analysis, we merged corticosteroids, steroids (> 0.5 mg/kg prednisone equivalents) and steroids (≤ 0.5 mg/kg prednisone equivalents) into one variable “steroids”.
Comorbidities
Comorbidities were self-reported physician-diagnosed diseases. (Detailed in Table 1).
Statistical analysis
Mean, with standard deviation (SD), or median with quartiles were used for the description of continuous variables. Counts and percentages were used for the description of categorical variables.
In the survival analysis, being symptom-free served as the event and time to be symptom-free as the time variable. Since < 50% of symptomatic participants were symptom-free at the time of investigation, we reported the Q1 (25%) time to symptom-free, instead of the median time. Kaplan–Meier estimator served to estimate the survival function and Kaplan–Meier plots served to visualize the survival curves. Log-rank tests were used to test group differences in both overall survival curves and in survival curves up to 28 days.
Missing data were imputed by Multiple Imputation by Chained Equations (MICE) [28], yielding ten imputed datasets. Imputation was based on age, sex, educational level, living status, smoking, alcohol consumption, symptom burden during acute infection, BMI, COVID-19 treatment during acute infection, chronic liver disease, chronic rheumatologic/immunologic disease, tumor/cancer disease, chronic neurological disease, lung disease, ear, nose and throat (ENT) disease, cardiovascular disease, and diabetes. The final model was combined with Rubin’s rules, calculating final coefficient as the mean of coefficients estimated from imputed datasets and calculating the variance of estimated coefficients by factoring in the within and between imputation variance [29].
We applied a stratified Cox proportional hazard regression model to explore the factors predicting prolonged time to symptom-free after infection. Proportional hazard (PH) assumption was assessed with the Schoenfeld test [30]. Predictors violating the PH assumption were included as a stratified parameter in the multivariable Cox model [30]. By including a variable as a stratified parameter, the stratified Cox proportional hazard model sets a different baseline hazard corresponding to each stratum as defined by the variable, and then estimates common coefficients for the remaining explanatory variables except for the stratified variable, thus providing hazard ratios controlled for the effect of the stratification variable, but not for the stratification variable itself [30]. Symptom burden and hospitalization both violated the PH assumption and both are closely related to unmeasured disease severity during the acute infection phase. Since only 75 (6.4%) of all patients were hospitalized, we decided to only include symptom burden as a stratification parameter and analyzed the effect of hospitalization in a separate sensitivity analysis (see below). A Generalized Variance Inflation Factor (GVIF) was used to check for multicollinearity among covariates, GVIF1/(2*Df) of ≥ 5 was considered indicative of collinearity [31]. Stepwise variable selection was conducted, selecting the model with the smallest Akaike information criterion. To assess the linearity assumption, we plotted the Martingale residuals against covariates. The adjusted hazard ratios (aHRs) were used to describe the hazard of becoming symptom-free, with aHR < 1 indicating a longer time to symptom free. A multivariate Wald test was used to assess the overall significance of difference for categorical variables with more than three categories. The concordance index (C-index) was used to measure the goodness-of-fit of the fitted models with ten imputed datasets; it measures the agreement between observed survival and predicted survival, with a value of 0.5 representing a random prediction and a value of 1.0 representing the best possible model prediction [32].
The threshold for statistical significance was set to 0.05. Since this was an exploratory study, no correction for multiple testing was applied. We used R (version 4.1.1) with the dplyr, survival, car, MASS, and mice packages for all statistical analyses. MS Office and R were used to create figures.
Sensitivity analyses
To evaluate the robustness of the final model, we conducted separate Cox proportional hazard models for each potential risk factor adjusted for age and sex. To investigate the effect of hospitalization on time to symptom-free we conducted three separate models: the first model only for patients having been hospitalized during acute infection, the second model for patients not having been hospitalized, and the third model including hospitalization with two different effect estimates, one for the effect in the first four weeks and one afterwards.
Results
Study participants
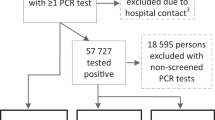
Data from 1441 COVIDOM/NAPKON-POP participants were available, including 1126 from Kiel, 208 from Würzburg, and 107 from Berlin. After excluding 90 cases with a time between PCR test and survey of < 6 months, and one case with an implausible PCR test date, 1350 participants were eligible for the present analysis. Of these, 108 participants had been asymptomatic during the acute phase, information on the current symptom status or the time to symptom-free of another 67 participants were missing. They were thus excluded from the analyses, resulting in a final sample of 1175 participants (Fig. 2).
Mean time since the onset of infection for 1175 participants was 280 days (SD 68). 54.1% of initially symptomatic participants continued to experience symptoms. Sex, BMI, resilience and most comorbidities of symptomatic participants were comparable to asymptomatic participants, whereas age, nationality, educational level, living status, smoking status, and COVID-19 treatment were not (Table 1).
Persistent COVID-19-related symptoms
At the time of survey, 22 of 23 different symptoms from the acute phase were still persistent: anosmia (19.3%), dyspnea (18.9%), fatigue (14.1%), and ageusia (13.8%) were the most common persisting symptoms. Muscle pain, headache, limb pain, dizziness, disturbances of consciousness/confusion, chest pain, and cough were reported by > 5% of participants each. Over 40% of participants had suffered from sore throat, fever, chills, and a runny nose during acute infection, while only < 5% reported these symptoms at the time of the survey, respectively (Fig. 1).
Time to symptom-free
Figure 3 and Table 2 summarize the observed bivariate differences in symptom persistence. Q1 time to symptom-free was 18 days [quartiles: 14 days, 21 days]. 405 (34.5%) participants had become symptom-free during the first 28 days since symptom onset, and only slow symptom resolution was seen afterwards. Time to symptom-free differed according to age, sex, educational level, living status, alcohol consumption, hospitalization during acute infection, symptom burden during acute infection, BMI, resilience, steroid treatment during acute infection, chronic liver disease, chronic rheumatologic/immunologic disease, chronic neurological disease, lung disease, and cardiovascular disease. Similar results were obtained when testing for group differences in survival curves up to 28 days, except for living status, smoking status, alcohol consumption, BMI, anticoagulation treatment and lung disease.
Prognostic analyses
Symptom burden during acute infection was included as a stratification variable in the final model because it violated the PH assumption. All GVIF were smaller than 5. Other variables included in the final model were age, sex, educational level, living status, alcohol consumption, BMI, resilience, COVID-19 medication and steroid treatment during acute infection, chronic liver disease, chronic rheumatologic/immunologic disease, and chronic neurological disease. The concordance indices of the ten fitted models ranged between 0.6305 and 0.6401.
Patients aged 49–59 years had a 30% lower hazard of becoming symptom-free than those aged < 49 years (aHR 0.70, 95% CI 0.56–0.87), while the hazard for patients ≥ 60 years did not differ from that < 49 years. Prolonged time to recovery was also seen in women (aHR 0.78, 95% CI 0.65–0.93), and patients with lower educational level (aHR 0.77, 95% CI 0.64–0.93), or living with a partner (aHR 0.81, 95% CI 0.66–0.99), or with low resilience (aHR 0.65, 95% CI 0.47–0.90). Steroid treatment (aHR 0.22, 95% CI 0.05–0.90) and no medication (aHR 0.74, 95% CI 0.62–0.89) during acute infection also increased time to symptom-free (Table 3).
Age and sex-adjusted coefficients for each potential risk factor can be found in the Supplementary Appendix (S Table 2). Cox proportional hazard models for hospitalized patients and non-hospitalized patients, together with time-varying effect estimates of hospitalization can be found in the Supplementary Appendix (S Table 3–5). Non-hospitalized patients were more likely to become symptom-free in the first four weeks (aHR 2.42, 95% CI 1.28–4.59). No significant differences were found after this time period.
Discussion
Main findings
We used data from a large population-based multicenter study for the retrospective analysis of the duration of, and risk factors for a prolonged recovery from acute SARS-CoV-2 infection. While 65.5% of included participants reported to still have symptoms 28 days after infection, over half of the symptomatic participants (54.1%) experienced at least one persisting symptom about 9 months post-infection. 22 of 23 different symptoms during the acute phase except for vomiting persisted beyond 9 months, with anosmia, dyspnea, ageusia, and fatigue being the most frequent ones. We found that female sex, age between 49 and 59 years, lower educational level, living with a partner, low resilience, steroid treatment and no medication during acute infection were associated with prolonged time to symptom-free, and being hospitalized was associated with prolonged time only in the first four weeks.
Study findings in context
We found that COVID-19-related symptoms rapidly resolved at the beginning but only incremental improvement was seen beyond 28 days. A former study also demonstrated that symptom load at 1.5 to 6 months was not associated with the length of time since symptom onset, suggesting that improvement in symptoms primarily occurred during the first few weeks after infection [12]. Furthermore, most subgroup differences in time to symptom-free occurred within 28 days after symptom onset in our study.
The most prevalent symptoms including anosmia, dyspnea, ageusia, and fatigue corresponded to those reported in a study of non-hospitalized individuals and another one of patients with mild or moderate symptoms [12, 16]. Long persistence of symptoms is worrying because persisting COVID-19 symptoms are associated with poor health-related quality of life (HRQOL) [9, 33]. Even though the present analysis did not differentiate symptoms according to their severity or their impact on daily life or HRQOL, our previous analysis of COVIDOM/NAPKON-POP data [9] revealed that different symptoms have a different impact on the severity of PCS and, consequently, on HRQOL. Therefore, learning more about symptom persistence and symptom resolution is of utmost clinical relevance.
Our study identified several risk factors for prolonged symptom persistence. An age between 49 and 59 years, being female, lower education, living with a partner, low resilience, steroid treatment, and no medication during acute infection were factors that predicted longer symptom persistence. Some of these factors like age are in line with previous studies [21, 34], although the inverse U-shaped association of age with risk might seem surprising. However, similar results were obtained from 10 longitudinal studies in the UK, with the highest risk noted in the middle age categories, i.e. 45–54 and 55–69 years [20]. Arguably, this might be attributable to competing mortality risks or erroneous attribution of symptoms to other causes in older age [20]. On the other hand, we cannot exclude that participants’ differential recall might also have been determined by some of the risk factors in question, especially age, resilience, and education. Hence, the identified predictors still require confirmation by independent longitudinal studies. Consistent with most previous studies [21, 23, 35, 36], we found that female patients were less likely to recover quickly from symptoms than male patients. In contrast to our results, a Swedish study found that the female sex was protective for Long COVID-related sick leave, but only in a subgroup of hospitalized patients [37]. Patients with lower education are more likely to have physically demanding jobs [38], which might have influenced their recovery from symptoms. The effect of living status might be due to recall bias since patients living with a partner might have discussed their symptoms more frequently with their partner, as compared to patients without a partner or not living with a partner. This might result in differential reporting of symptoms in patients without a partner or not living with a partner, thus the observed effect should be interpreted with caution. Moreover, it may be speculated that constant exposure to a partner’s infection might have increased virus load. In our previous study [9], we found low resilience and strong acute disease severity to be risk factors for severe PCS. Similarly, patients with more severe acute COVID-19 were also reported to show prolonged symptoms [39]. Likewise, steroid treatment might be an indicator of disease severity that results in prolonged symptoms. Although it has been shown that inhaled corticosteroid treatment improved symptom resolution in COVID-19 patients [40], a meta-analysis demonstrated an association between corticosteroid therapy and increased length of stay, although this finding was only based on subgroup analysis in three randomized controlled trials [41].
Strengths and limitations
A major strength of our study is that we reported a population-based estimate of the status and duration of symptoms drawing upon data from over 1100 COVID-19 patients with an average follow-up of 9 months.
There are some limitations. First and foremost, our use of the COVIDOM/NAPKON-POP time-to-recovery data had to be retrospective in nature because the study did not collect symptoms prospectively starting from infection. Since this might have been subject to recall bias, factors affecting the precision of the derived time-to-recovery data might have confounded some of the relationships between the latter and potential predictors. However, it is also likely that patients remember the time course well even after recovery. Second, as this study is not a representative sample of the total population, selection bias must be taken into account. It has to be mentioned that selection and differential response could have biased the estimates of the prevalence and persistence of symptoms. However, given the nature of the cooperation with the local health authorities, we are confident that the COVIDOM/NAPKON-POP sample is a valid representation of the infected population at the given time in the respective regions. Third, symptom status was collected by self-report, asking participants about COVID-19-related symptoms. However, we cannot rule out the possibility that some symptoms were caused by other respiratory infections. Furthermore, although we assume that most participants would not mention a chronic symptom as it is not noticeably related to the COVID-19 disease, future studies should evaluate the presence of symptoms before COVID-19 and their potential aggravation because of COVID-19. Fourth, long-term symptom status of initially asymptomatic patients was not evaluated. It is still unknown whether this group developed new symptoms after acute infection. Third, patients included in COVIDOM/NAPKON study probably mainly had SARS-CoV-2 wild type or alpha variant infection with a higher burden of symptoms than later variants. Future analyses of the cohort population from 2022 will evaluate how comparable symptom persistence after the omicron variant is to our present findings. Finally, the study does not include a control group, which makes it difficult to know whether the reported symptoms can indeed be attributed to SARS-CoV-2 infection.
Conclusions
Over half of the participants reported COVID-19-related symptoms 9 months after infection. Many patients experienced rapid recovery, but prolonged recovery was also seen particularly among those characterized by middle age, female sex, lower educational level, living with a partner, low resilience, and without medication during acute infection.
Data availability
Data of this study are available upon request to the Use & Access Committee (UAC) of NAPKON (https://proskive.napkon.de).
References
World Health Organization. WHO coronavirus (COVID-19) dashboard.
Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6:e005427. https://doi.org/10.1136/bmjgh-2021-005427.
van Kessel SAM, Olde Hartman TC, Lucassen PLBJ, van Jaarsveld CHM. Post-acute and long-COVID-19 symptoms in patients with mild diseases: a systematic review. Fam Pract. 2021;39:159–67. https://doi.org/10.1093/fampra/cmab076.
National Institute for Health and Care Excellence: Clinical Guidelines. COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Institute for Health and Care Excellence (NICE); 2022.
Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:657–66. https://doi.org/10.1016/j.cmi.2022.01.014.
Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4:e2111417. https://doi.org/10.1001/jamanetworkopen.2021.11417.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. https://doi.org/10.1038/s41598-021-95565-8.
Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114:428–42. https://doi.org/10.1177/01410768211032850.
Bahmer T, Borzikowsky C, Lieb W, Horn A, Krist L, Fricke J, et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: a prospective, multi-centre, population-based cohort study. EClinicalMedicine. 2022;51:101549. https://doi.org/10.1016/j.eclinm.2022.101549.
Ballering AV, van Zon SKR, olde Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452–61. https://doi.org/10.1016/S0140-6736(22)01214-4.
Zeng N, Zhao Y-M, Yan W, Li C, Lu Q-D, Liu L, et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psychiatry. 2023;28:423–33. https://doi.org/10.1038/s41380-022-01614-7.
Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2021;76:405–7. https://doi.org/10.1136/thoraxjnl-2020-216377.
Hirschtick JL, Titus AR, Slocum E, Power LE, Hirschtick RE, Elliott MR, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis. 2021;73:2055–64. https://doi.org/10.1093/cid/ciab408.
Blomberg B, Mohn KG-I, Brokstad KA, Zhou F, Linchausen DW, Hansen B-A, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–13. https://doi.org/10.1038/s41591-021-01433-3.
Petersen MS, Kristiansen MF, Hanusson KD, Danielsen ME, á Steig B, Gaini S, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2020;73:e4058–63. https://doi.org/10.1093/cid/ciaa1792.
Boscolo-Rizzo P, Guida F, Polesel J, Marcuzzo AV, Capriotti V, D’Alessandro A, et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol. 2021;11:1685–8. https://doi.org/10.1002/alr.22832.
Wynberg E, van Willigen HDG, Dijkstra M, Boyd A, Kootstra NA, van den Aardweg JG, et al. Evolution of Coronavirus Disease 2019 (COVID-19) symptoms during the first 12 months after illness onset. Clin Infect Dis. 2022;75:e482–90. https://doi.org/10.1093/cid/ciab759.
Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. https://doi.org/10.1016/j.eclinm.2021.101019.
The PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. 2022;10:761–75. https://doi.org/10.1016/s2213-2600(22)00127-8.
Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13:3528. https://doi.org/10.1038/s41467-022-30836-0.
Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–31. https://doi.org/10.1038/s41591-021-01292-y.
Wu Q, Ailshire JA, Crimmins EM. Long COVID and symptom trajectory in a representative sample of Americans in the first year of the pandemic. Sci Rep. 2022;12:11647. https://doi.org/10.1038/s41598-022-15727-0.
Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–87. https://doi.org/10.1016/S2213-2600(21)00383-0.
Schons M, Pilgram L, Reese J-P, Stecher M, Anton G, Appel KS, et al. The German National Pandemic Cohort Network (NAPKON): rationale, study design and baseline characteristics. Eur J Epidemiol. 2022;37(8):849–70. https://doi.org/10.1007/s10654-022-00896-z.
Horn A, Krist L, Lieb W, Montellano FA, Kohls M, Haas K, et al. Long-term health sequelae and quality of life at least 6 months after infection with SARS-CoV-2: design and rationale of the COVIDOM-study as part of the NAPKON population-based cohort platform (POP). Infection. 2021;49:1277–87. https://doi.org/10.1007/s15010-021-01707-5.
Federal Center for Health Education, German Medical Association. Addressing alcohol consumption in patients. Medical manual for the prevention and treatment of risky, harmful and dependent consumption. 2021.
WHO Consultation on Obesity, World Health Organization. Obesity : preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization; 2000.
Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–9. https://doi.org/10.1002/mpr.329.
Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 2004.
Kleinbaum DG, Klein M. Survival analysis: a self-learning text. Berlin: Springer; 2012.
Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87(417):178–83. https://doi.org/10.1080/01621459.1992.10475190.
Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. https://doi.org/10.1001/jama.1982.03320430047030.
Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—a systematic review and meta-analysis. J Med Virol. 2022;94:253–62. https://doi.org/10.1002/jmv.27309.
Kostev K, Smith L, Koyanagi A, Jacob L. Prevalence of and factors associated with post-coronavirus disease 2019 (COVID-19) condition in the 12 months after the diagnosis of COVID-19 in adults followed in general practices in Germany. Open Forum Infect Dis. 2022;9:333. https://doi.org/10.1093/ofid/ofac333.
Peghin M, Palese A, Venturini M, De Martino M, Gerussi V, Graziano E, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27:1507–13. https://doi.org/10.1016/j.cmi.2021.05.033.
Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28:1706–14. https://doi.org/10.1038/s41591-022-01909-w.
Westerlind E, Palstam A, Sunnerhagen KS, Persson HC. Patterns and predictors of sick leave after Covid-19 and long Covid in a national Swedish cohort. BMC Public Health. 2021;21:1023. https://doi.org/10.1186/s12889-021-11013-2.
Warren JR, Hoonakker P, Carayon P, Brand J. Job characteristics as mediators in SES–health relationships. Soc Sci Med. 2004;59:1367–78. https://doi.org/10.1016/j.socscimed.2004.01.035.
Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021;75:e13746. https://doi.org/10.1111/ijcp.13746.
Chen CH, Wang CY, Wang YH, Chen CY, Chen KH, Lai CC, et al. The effect of inhaled corticosteroids on the outcomes of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol. 2022;15:593–600. https://doi.org/10.1080/17512433.2022.2094769.
Zhou F, Deng J, Heybati K, Zuo QK, Ali S, Hou W, et al. Efficacy and safety of corticosteroid regimens for the treatment of hospitalized COVID-19 patients: a meta-analysis. Future Virol. 2022;17:463–89. https://doi.org/10.2217/fvl-2021-0244.
Acknowledgements
We acknowledge Dr. Nicole S. Erler from Erasmus University Medical Center for assisting with multiple imputations. Data were provided by NAPKON (Nationales Pandemie Kohorten Netz, German National Pandemic Cohort Network) of the Network University Medicine (NUM), funded by the Federal Ministry of Education and Research (BMBF).
We express our gratitude to all collaborators of the NAPKON study group, including study teams, the governance bodies, individuals who made contributions to coordination and infrastructure of NAPKON and the coordination of NUM, and the clinical research support staff. The NAPKON study team members include: Agaplesion Bethesda Krankenhaus Bergedorf gemeinnützige GmbH (Marc Gregor Bota), Elisabeth-Krankenhaus Essen (Ingo Voigt), Frankfurt Universitätsklinikum (Maria J.G.T. Vehreschild, Jörg J. Vehreschild), Hausarztpraxis Dashti (Hiwa Dashti), Hausarztpraxis Egestorf, Bispingen (Barbara Laumerich), Helios Klinik Bad Saarow (Oliver Pociuli), Helios Klinikum Duisburg (Nikolaus Büchner), Helios Klinikum Erfurt (Sabine Adler), Helios Klinikum Krefeld (Mathias Lehmann), Helios Klinikum Siegburg (Selcuk Tasci), Kinder- und Jugendpraxis Jorczyk, Dresden (Maximilian Jorczyk), KJF Klinik Josefinum gGmbH (Thomas Keller), Klinik Hallerwiese-Cnopfsche Kinderklinik (Michael Schroth), Klinikum Dortmund gGmbH, Medizinische Klinik Nord (Martin Hower), Klinikum Leverkusen (Lukas Eberwein), Klinikum Worms (Tim Zimmermann), Krankenhaus Bethanien—Institut für Pneumologie an der Universität zu Köln (Simon-Dominik Herkenrath), Malteser Krankenhaus St. Franziskus-Hospital (SFH) Flensburg (Milena Milovanovic), MVZ am Isartor, München (Ramona Pauli), MVZ im Altstadt-Caree Fulda GmbH Medizinisches Versorgungszentrum (Jörg Simon), OWL: Evangelisches Klinikum Bethel (Eckard Hamelmann), OWL: Klinikum Bielefeld (Christoph Stellbrink), OWL: Klinikum Lippe (Johannes-Josef Tebbe), Petrus Krankenhaus Wuppertal (Sven Stieglitz), Praxis am Ebertplatz (Christoph Wyen), Praxis Bosch und Renner, Köln (Jan Bosch), Praxis Dilltal, Burbach (Mirko Steinmüller), Praxis Dr. Allerlei, Frankfurt am Main (Christoph Allerlei), Praxis Dr. Böbel, Reutlingen (Markus Böbel), Praxis Dr. Elke Heinitz, Grube (Elke Natascha Heinitz), Praxis Dr. med Ariane Roecken (Ariane Roecken), Praxis Dr. med. Andrea Münkle-Krimly (Andrea Münckle-Krimly), Praxis Dr. med. Christiane Guderian (Christiane Guderian), Praxis im Tennental (Ingmar Silberbaur), SHG-Kliniken Völklingen (Harald Schäfer), Tropenklinik Paul-Lechler-Krankenhaus Tübingen (Claudia Raichle), TUM München (Christoph Spinner), UKGM Standort Marburg (Bernd Schmeck), Uniklinik Carl Gustav Carus Dresden (Heidi Altmann, Nicole Toepfner), Uniklinik der Ruhr-Universität Bochum (Wolfgang Schmidt), Uniklinik Düsseldorf (Björn Jensen), Uniklinik Erlangen (Andreas Kremer), Uniklinik Göttingen (Sabine Blaschke), Uniklinik Halle (Jochen Dutzmann), Uniklinik Hamburg-Eppendorf (Marylyn Addo), Uniklinik Homburg (Robert Bals), Uniklinik Leipzig (Sven Bercker), Uniklinik Münster (Phil-Robin Tepasse), Uniklinik Regensburg (Frank Hanses), Uniklinik RWTH Aachen (Dirk Müller-Wieland), Uniklinik Schleswig-Holstein, Kiel (Anette Friedrichs), Uniklinik Schleswig-Holstein, Lübeck (Jan Rupp), Uniklinik Tübingen (Siri Göpel), Uniklinik Würzburg (Jens Maschmann), Universitätsklinikum Augsburg (Christine Dhillon), Universitätsklinikum Bonn (Jacob Nattermann), Universitätsklinikum Essen (Ingo Voigt), Universitätsklinikum Magdeburg (Wilfred Obst), Universitätsmedizin der Johannes Gutenberg-Universität Mainz (Martin Franz Sprinzl), Universitätsmedizin Greifswald (Christian Scheer), Universitätsmedizin Mannheim (Andreas Teufel), Universitätsmedizin Oldenburg (Ulf Günther), Charité—Universitätsmedizin Berlin (Martin Witzenrath, Thomas Keil, Thomas Zoller, Sein Schmidt, Michael Hummel, Lilian Krist, Julia Fricke, Maria Rönnefarth, Denise Treue, Ludie Kretzler, Chantip Dang-Heine, Paul Triller, Andreas Jooß, Jenny Schlesinger, Natalja Liseweski, Christina Pley, Carmen Scheibenbogen), Hannover Medizinische Hochschule (Marius Hoeper), Jena (Philipp A. Reuken), Klinikum der Universität München (Michael von Bergwelt), UKSH-Kiel (Rainer Noth), UKSH-Lübeck (Daniel Drömann), Universitätsklinikum Frankfurt (Maria J.G.T. Vehreschild), Universitätsklinikum Freiburg (Siegbert Rieg), Universitätsklinikum Giessen / Marburg (Istvan Vadasz), Universitätsklinikum Köln (Philipp A. Koehler), Universtitästklinikum Heidelberg (Uta Merle), Kiel Universitätsklinikum (Stefan Schreiber), Würzburg Universitätsklinikum (Peter Heuschmann, Stefan Störk). The following individuals have supported governance bodies of the NAPKON: Use & Access Committee Anette Friedrichs, Astrid Petersmann, Claudia Ellert, Georg Schmidt, Janne Vehreschild, Katrin Milger, Marie von Lilienfeld, Martin Witzenrath, Oliver Witzke, Patrick Meybohm, Peter Heuschmann, Sabine Blaschke, Sandra Frank, Stefan Schreiber, Thomas Illig. Advisory Board Alexander Hein, Andrea Wittig, Andreas Simm, Anette Friedrichs, Anke Reinacher-Schick, Anna Frey, Antonella Iannaccone, Astrid Petersmann, Benjamin Maasoumy, Benjamin Waschki, Bimba Hoyer, Brigitt van Oorschot, Carolina van Schaik, Christina Lemhöfer, Christina Polidori, Christine Klein, Daniel Medenwald, Eva Christina Schulte, Eva Grill, Felix Meinel, Folke Brinkmann, Ghazal Arabi, Heike Bickeböller, Holger Lindner, Ildiko Gagyor, Jessica Hassel, Jürgen Deckert, Katrin Milger-Kneidinger, Kerstin Ludwig, Marcus Dörr, Marie von Lilienfeld-Toal, Martin Möckel, Martin Weigl, Matthias Nauck, Miriam Banas, Muenevver Demir, Nicole Lindenberg, Nora Hettich, Norma Jung, Oliver Witzke, Orlando Guntinas-Lichius, Patrick Meybohm, Reinhard Berner, Sabine Blaschke, Samuel Knauss, Sandra Frank, Sebastian Baumeister, Sebastian Dolff, Selma Ugurel, Sophia Stöcklein, Stefanie Joos, Winfred Häuser. The coordination and infrastructure of the NAPKON was conducted by: ICU: Jörg Janne Vehreschild, Maximilian Schons, Sina Hopff, Markus Brechtel, Cristina Schmidt-Ibanez, Johannes Schneider, Carolin Jakob, Franziska Voß. BCU: Inga Bernemann, Sonja Kunze, Maike Tauchert, Thomas Illig, Gabriele Anton. ECU: Cornelia Fiessler, Mirjam Kohls, Olga Miljukov, Steffi Jiru-Hillmann, Jens-Peter Reese, Peter Heuschmann. IGCU: Jens-Peter Reese, Peter Heuschmann, Anna-Lena Hofmann, Julia Schmidt, Kathrin Ungethüm, Anna Horn, Michael Krawczak. POP: Thomas Bahmer, Wolfgang Lieb, Daniel Pape, Stefan Schreiber, Anne Hermes, Irene Lehmann, Corina Maetzler, Lukas Tittmann. DZHK: Roberto Lorbeer, Bettina Lorenz-Depiereux, Monika Kraus, Christian Schäfer, Jens Schaller, Mario Schattschneider, Dana Stahl, Heike Valentin, Dagmar Krefting, Matthias Nauck. Pediatric core unit: Nicole Toepfner, Reinhard Berner. GECCO & Interoperability Team: Christof von Kalle, Sylvia Thun, Alexander Bartschke, Liudmila Lysyakova, Stefanie Rudolph, Julian Sass. Kardiology team: Eike Nagel, Valentina Püntmann. We acknowledge the dedicated support of clinical research support staff of the Institute of Experimental and Translational Cardiovascular Imaging, including Tammy Wolf, Thourier Azdad, Franziska Weis, Ira Krückemeier, Simon Bohlender, Deniz Desik, and Layla Laghchioua. Coordination NUM: Ralf Heyder, Silke Wiedmann.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Federal Ministry of Education and Research (BMBF) via the Network University Medicine (FKZ: 01KX2021). Parts of the infrastructure of the Kiel and Würzburg study sites were supported by the federal states of Schleswig–Holstein and Bavaria. Yanyan Shi is supported by a scholarship from the China Scholarship Council (202108140048).
Author information
Authors and Affiliations
Consortia
Contributions
YS: statistical analysis; interpretation of results; drafting and revising the manuscript. RS: acquisition and interpretation of data; statistical support; revision of the draft. JPR, FAM: acquisition and interpretation of data; revision of the draft. SSch, TB, WL, MK, PH, SStö, AH, LK, TK, MW: conceptualization of the COVIDOM/NAPKON-POP study; acquisition and interpretation of data; revision of the draft. JJV, RG, NS, RM, HH, BLD, CL, CA: revision of the draft. EG: conception and design of the present study; acquisition and interpretation of data; revision of the draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
TB reports personal fees from AstraZeneca, GlaxoSmithKline, Novartis, Roche, Chiesi, Boehringer Ingelheim, MSD and Pfizer outside the submitted work. SStö reports research grants of the Federal Ministry of Education and Research (#01EO1004; #01EO1504), speaker honoraria or advisory board honoraria of AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, and case payment fees in clinical studies of Alnylam, AstraZeneca, Boehringer Ingelheim, IONIS, MSD, NovoNordisk, SOBI, Servier; all outside the submitted work. All other authors have no competing interests to declare.
Ethical approval
COVIDOM/NAPKON-POP is registered at the German Registry for clinical studies (DRKS00023742) and at http://www.clinicaltrials.gov (NCT04679584). NAPKON-POP was approved by the local ethic committees of respective study sites (Kiel, No. D 537/20; Würzburg, No. 236/20_z). According to the professional code of the Berlin Medical Association regarding multi-center studies, approval by the Ethics Committee of the coordinating study center (Kiel) was also valid for the Berlin study site [25]. Written informed consent was obtained prior to all procedures from all participants.
Additional information
The members of the NAPKON Study Group are listed in Acknowledgements.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, Y., Strobl, R., Apfelbacher, C. et al. Persistent symptoms and risk factors predicting prolonged time to symptom-free after SARS‑CoV‑2 infection: an analysis of the baseline examination of the German COVIDOM/NAPKON-POP cohort. Infection 51, 1679–1694 (2023). https://doi.org/10.1007/s15010-023-02043-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-02043-6