Abstract
Purpose
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is currently the major threat for immunocompromised individuals. The course of COVID-19 in lung transplant recipients in the Omicron era remains unknown. The aim of the study was to assess outcome and associated factors in lung transplant recipients in a German-wide multicenter approach.
Methods
All affected individuals from January 1st to March 20th, 2022 from 8 German centers during the Omicron wave were collected. Baseline characteristics and antiviral measures were associated with outcome.
Results
Of 218 patients with PCR-proven SARS-CoV-2 infection 166 patients (76%) received any early (< 7 days) antiviral therapy median 2 (interquartile range 1–4) days after symptom onset. Most patients received sotrovimab (57%), followed by remdesivir (21%) and molnupiravir (21%). An early combination therapy was applied in 45 patients (21%). Thirty-four patients (16%) developed a severe or critical disease severity according to the WHO scale. In total, 14 patients (6.4%) died subsequently associated with COVID-19. Neither vaccination and antibody status, nor applied treatments were associated with outcome. Only age and glomerular filtration rate < 30 ml/min/1.73m2 were independent risk factors for a severe or critical COVID-19.
Conclusion
COVID-19 due to Omicron remains an important threat for lung transplant recipients. In particular, elderly patients and patients with impaired kidney function are at risk for worse outcome. Prophylaxis and therapy in highly immunocompromised individuals need further improvement.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is currently the major threat for immunocompromised individuals. Lung transplant recipients are believed to be at particular risk for worse outcome due to the high dose of immunosuppressive drugs and the lung as the main organ affected by COVID-19 [1, 2].
Before the use of active immunization and other antiviral measures, 30-day mortality rates of lung transplant recipients with COVID-19 of 30–40% were reported [3, 4]. Later during the pandemic both hospitalization and mortality rates have declined, [5] which has been associated with vaccination rates [6] and early application of monoclonal antibodies (mAbs) [7]. However, mortality remains significant and long-term sequelae remain unknown.
At the end of 2021, the new variant of concern (VOC) Omicron (B.1.1.529) has become the dominant SARS-CoV-2 strain globally. High transmissibility due to the ability to evade SARS-CoV-2 immunity acquired by either vaccination or past infection [8, 9] and likely other intrinsic virological properties, like faster replication in the upper airways resulted in a dramatic increase of affected individuals and the largest wave since the beginning of the pandemic [10]. Yet, Omicron-infected individuals have significantly reduced odds of severe disease compared with individuals infected earlier with the VOC Delta (B.1.617.2) [11]. However, in patients requiring hospitalization disease severity seems to be comparable to Delta [12].
Furthermore, concomitantly to the appearance of Omicron a variety of antiviral treatments became available. The mAb sotrovimab with neutralizing activity against Omicron [13, 14] and the antiviral drug molnupiravir alone [15] or in combination with other antiviral measures have been widely accepted as standard of care particularly in unvaccinated and immunocompromised patients with COVID-19.
However, despite potentially milder disease and new antiviral measures the outcome of COVID-19 in lung transplant recipients remains unknown. In addition to the immune escape of Omicron, the antibody response after vaccination is frequently insufficient in immunocompromised individuals mitigating the beneficial effect of a complete primary series and booster doses of vaccines [16]. Furthermore, antiviral drugs and combinations thereof have not been studied in transplant recipients.
Therefore, the course of COVID-19 in lung transplant recipients in the Omicron era remains unknown. The aim of this study was to assess outcome and associated factors of patients after lung transplantation with COVID-19 in a German-wide initiative during the recent Omicron wave.
Methods
All lung transplant recipients with polymerase chain reaction (PCR)-proven SARS-CoV-2 infection from January 1st to March 20th, 2022 from 8 German lung transplant centers (Berlin, Essen, Freiburg, Giessen, Hamburg, Hannover, Homburg, Leipzig and Munich) were included in the study. The study was approved by the central institutional ethics committee (Munich, Germany; project number 22-0078). This retrospective study was performed in accordance with the ethical guidelines of the 2000 Declaration of Helsinki and the standards of the 2008 Declaration of Istanbul.
In the beginning of January, Omicron (B.1.1.529) became the dominant VOC in Germany. To limit the analysis to patients with Omicron (B.1.1.529), lung transplant recipients with SARS-CoV-2 infection from January 1st to 31st were only included if SARS-CoV-2 VOC characterization was available and Omicron (B.1.1.529) detected. From 1st of February on, when Omicron (B.1.1.529) was almost exclusively (> 98%) present in Germany all affected individuals were included, if no other VOC was detected. If Omicron sublineages BA.1 and BA.2 were not recorded, patients with SARS-CoV-2 infection before February 21st were attributed to BA.1. Thereafter, patients were attributed to BA.2 according to the dominant occurrence of sublineages in Germany.
Baseline characteristics (age, sex, underlying disease, lung transplant procedure: bilateral, unilateral or combined organ transplantation and immunosuppressive regimen) and comorbidities (diabetes mellitus, obesity (defined as body mass index > 30 kg/m2), chronic lung allograft dysfunction (CLAD), chronic kidney disease (i.e., glomerular filtration rate (GFR) < 30 ml/min/1.73m2) and coronary artery disease were recorded. CLAD was defined according to the established criteria [17].
Furthermore, number and dates of previous vaccinations, type of vaccine, previous infections with SARS-CoV-2 and last available antibodies against Spike-protein (SARS-CoV-2-S) were recorded. A complete primary series of vaccination was defined as 3 doses of either mRNA-vaccines (BNT162b2/BioNTech/Pfizer, mRNA-1273/Moderna), 3 doses of ChAdOx1/AstraZeneca, combinations thereof or one dose Ad26.COV2.S/Johnson & Johnson a second dose of either mRNA COVID-19 vaccines. More vaccinations were defined as a complete primary series plus booster vaccination. SARS-CoV-2-S antibody status was classified in unknown, negative (binding antibody units (BAU) < 50/ml), low (BAU 50–250/ml) and positive (BAU > 250/ml).
Dates of symptom onset, SARS-CoV-2 infection proven by PCR and applied treatments, respectively, were recorded. Available antiviral treatments during the study periods were casirivimab/imdevimab, sotrovimab, remdesivir, molnupiravir and since the end of February nirmatrelvir/ritonavir. Antiviral treatments were regarded as early if started within less than 7 days after symptom onset. In the case of asymptomatic patients, the date of positive SARS-CoV-2 PCR was regarded as the date of disease onset. Other antiinflammatory treatments like dexamethasone and tocilizumab were recorded but not analyzed further due to their application only in advanced stages of disease.
COVID-19 severity was scored according to the WHO scale [18] with recording of the highest stage during follow-up after infection. In brief, mild disease was defined as constitutional symptoms without signs of pneumonia or respiratory failure. Moderate disease had signs of pneumonia without respiratory failure (blood oxygen saturation (SpO2) > 94%, no use of oxygen). Patients admitted for non-pulmonary manifestations were graded as moderate disease. Severe disease was defined as respiratory rate ≥ 30/min, SpO2 < 94%, use of oxygen or opacities > 50% on pulmonary imaging. Critical disease was defined as respiratory failure with need of mechanical ventilation support, presence of septic shock or multiple organ failure.
Follow-up after COVID-19 was recorded for at least 28 days or until death whichever occurred first.
Statistics
Metric variables were expressed as medians and interquartile range (IQR). Univariate analyses were performed using the Mann–Whitney test for continuous variables and chi-square test for categorical variables. Binary logistic regression analyses were conducted with severe or critical course of disease as the dependent variable. The level of significance was set at < 0.10 for including variables identified by univariate analysis between groups.
Results
In total, 218 patients were identified and included in the study. Patient characteristics are shown in Table 1. In brief, median age at time of COVID-19 was 56 (25, 75% percentile 45, 63) and 104 patients (48%) were female. Pre-existing CLAD was present in 53 individuals (24%) and comorbidities in descendent frequency were diabetes mellitus (n = 64, 29%), coronary artery disease (n = 35, 16%), chronic kidney disease (n = 32, 15%) and obesity (n = 12, 6%).
In 57 patients (26%), VOC was tested with confirmed Omicron BA.1; in 39 patients (68%) Omicron BA.2 in 18 patients (32%), respectively.
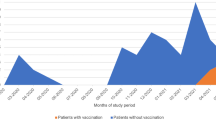
Vaccination status, antibody responses and vaccines used are shown in Fig. 1. A total of 188 patients (86%) had received a complete series of primary vaccines. Thereof, 44 patients (23%) received at least one booster vaccination. No vaccination was reported by 4 patients (2%). The most frequent vaccine was mRNA BNT162b2/BioNTech/Pfizer (80%). The majority of patients (70%) with known antibody status had a negative SARS-CoV-2-S antibody response. No patient with a negative antibody status had a low or positive antibody status in a previous assessment. No pre-exposure prophylaxis was used in patients.
Any early antiviral therapy (i.e., < 7 days of symptom onset) was given in 166 patients (76%) median 2 (IQR 1, 4) days after symptom onset. Of the remaining 52 patients (24%) 28 patients (14%) received treatment ≥ 7 days (median 9 days, IQR 7, 12) and 24 patients (12%) received no antiviral treatment at all. A combination of early antiviral treatments was given in 45 patients (21%). As shown in Table 2 early sotrovimab was the most frequent treatment (n = 125, 57%) followed by remdesivir (n = 46, 21%) and molnupiravir (n = 46, 21%). The applied treatments over the study period are shown in Fig. 2. No change in preferred treatment modalities was noted during the study period.
Disease severity is shown in Table 2. Most frequently, a mild course of disease (n = 137, 63%) was recorded. A total of 34 patients (16%) had a severe or critical course of disease. 15 patients (6.9%) died during follow-up and 14 deaths (6.4%) were attributed to COVID-19 median 27 (IQR 22, 33) days after disease onset.
In univariate regression analysis age at infection, time since lung transplantation and unilateral transplantation were associated with a severe or critical course of disease. Further associations were found for a GFR < 30 ml/min/1.73m2 and pre-existing CLAD. Immunosuppressive regime, vaccination and SARS-CoV-2-S antibody response and early antiviral therapies were not associated with a severe or critical course of disease (Table 3). In multivariate regression analysis, only age at infection (odds ratio (OR) 1.082, 95% CI 1.015–1.153; p = 0.015) and a GFR < 30 ml/min/1.73m2 (OR 3.175, 95% CI 1.278–7.884; p = 0.013) were associated with a severe or critical COVID-19 as shown in Table 3.
Discussion
In this multicenter national study during the Omicron (B.1.1.529) wave, one of six lung transplant recipients developed a severe or critical course of disease and COVID-19-associated mortality was 6.4%. Neither vaccination status and SARS-CoV-2-S antibody response, nor early antiviral treatments and combinations thereof seem to significantly impact the course of disease. Age and GFR < 30 ml/min/1.73m2 were independently associated with severe or critical COVID-19.
In contrast to previous reports of COVID-19 with other variants, the severity of disease in lung transplant recipients has decline; which is in line with previous reports on non-transplant individuals [11, 19, 20]. COVID-19-associated mortality has been reported of up to 30–40% in early single center studies and was 6.4% in our current analysis [3, 4]. Causes of more favorable course of COVID-19 with Omicron remain poorly understood. Omicron seems to have a tropism for the upper respiratory tract rather than the lower respiratory tract possibly leading to less COVID-19-associated pneumonias and therefore less severe courses of disease [19]. Furthermore, three exposures of vaccinations have been shown to effectively neutralize Omicron [16] which eventually results in less severe courses due to rapid viral clearance. However, the majority of our patients had no detectable SARS-CoV-2-S antibodies after several vaccinations, suggesting that active immunization may not be the only reason for the more favorable outcome. T-cell immunity was not assessed in our study, but it has been shown that the response is similarly attenuated [21, 22]. The poor vaccination response despite a complete primary series of vaccines and booster vaccinations is in line with previous reports on solid organ transplant recipients [21, 22].
Passive immunization by mAbs was the first antiviral treatment available. Dependent on the VOC mAbs were effective to a varying degree. Recently, we demonstrated in a cohort of lung transplant recipients with COVID-19 between the beginning of the pandemic and end of 2021 that the early application of casirivimab/imdevimab was associated with a significant survival benefit [7]. With Omicron casirivimab/imdevimab became ineffective. Another mAB sotrovimab was reported of having neutralizing activity in-vitro against Omicron and became available. Sotrovimab was the most frequently applied antiviral therapy in the current analysis. After increasing evidence on the efficacy of remdesivir in early COVID-19 [23] it became standard of care in many transplant centers. Additionally, in the beginning of our study molnupiravir became available and a beneficial side effect profile in transplant recipients was anticipated [15]. In contrast nirmatrelvir/ritonavir a highly CPY3A4-dependent drug leads to significant drug interactions [24] which likely was the main reason for sparse use in our study.
In a recently published publication UK-wide retrospective analysis in 142 SARS-CoV-2 (96% attributed to Omicron) infected kidney transplant recipients, 33% were treated with early Sotrovimab and 15% with early Molnupiravir. Admission rate was 21% in patients without early treatment and 1.4% died without early treatment. Use of sotrovimab was associated with lower admission rate (2%) indicating prevention of progression to severe disease in this study. In contrast to our study, early antiviral treatment was used less frequently (48 vs 76%) and severe and critical disease was reported infrequently in kidney transplant patients [25].
We focused on early treatments since antiviral therapy is most effective when viral replication takes place. Whether current or future antiviral measures in immunocompromised patients with prolonged and recurrent viral replication are of use was beyond the scope of our study. Furthermore, only early antiviral therapy was analyzed since we aimed to exclude a selection bias of patients in whom treatment was initiated when a severe course of disease was already present.
In the current analysis, early antiviral therapies seemed not to impact the course of COVID-19 significantly. This must be regarded with all limitations of our study. However, in contrast to the beneficial effect of casirivimab/imdevimab in lung transplant recipients with COVID-19 before Omicron becoming the dominant variant as reported previously [7], currently we were not able to demonstrate a similar association. Whether the neutralizing efficacy of sotrovimab for Omicron, or other Omicron specific factors are responsible for these observations remains unclear. Similarly, no association of early application of remdesivir and molnupiravir or combinations of different antiviral drugs with reduced odds for severe or critical courses of COVID-19 was found. In the setting of high degree immunosuppression with prolonged viral replication short-term antiviral attempts might, therefore, not be sufficient.
While sotrovimab retains in vitro activity against BA.1, a reduced activity against BA.2 has been debated [26,27,28]. It is currently unknown how this affects in vivo effectiveness of sotrovimab against BA.2. In multiple regression analysis, subvariant BA.1 or BA.2 was not associated with outcome, while rates of sotrovimab were similarly high.
Age and chronic kidney failure were independently associated with a worse outcome. Lung transplant recipients with a GFR < 30 ml/min/1.73m2 had three times increased risk for a severe or critical disease, highlighting the impact of kidney function on immune system in already immunocompromised patients. Age and comorbidities are known to be associated with worse outcome in patients with COVID-19 [29].
The results should be interpreted within their obvious limitations. This was a retrospective study with various antiviral therapies and combinations thereof applied. This is significantly in contrast to RCTs which are the standard to assess the efficacy of a therapeutic strategy. Our study should not change the present strategies to promote vaccination and should not prevent patients from getting antiviral therapies. Furthermore, the rapidly evolving field of COVID-19 makes a timely analysis difficult. The moving landscape of Omicron sublineages with the appearance of BA.4 and BA.5 and the increasing use of pre-exposure prophylaxis with tixagevimab and cilgavimab could not be addressed in this study. However, we believe that our observations on the impact of antiviral measures have highlighted the need for further preventive and therapeutic strategies. Course of COVID-19 was the primary outcome of the current study. In survivors, long-term sequela would be of interest but were beyond the scope of the study. Long-COVID and the impact of COVID-19 on CLAD development remain to be assessed.
In conclusion, our study demonstrates that in lung transplant recipients COVID-19 due to Omicron (B.1.1.529) has resulted in less severe cases and reduced mortality compared to previous variants. However, COVID-19 remains a significant threat for lung transplant recipients. Vaccination status with poor response and currently available early antiviral treatments were not associated with a reduced risk for severe or critical COVID-19. Advanced age and chronic kidney failure are risk factors for worse outcome. Additional antiviral measures are required for affected individuals.
Availability of data and materials
Data are available on reasonable request from the corresponding author.
Abbreviations
- BAU:
-
Binding antibody units
- CLAD:
-
Chronic lung allograft dysfunction
- COVID-19:
-
Coronavirus disease 2019
- GFR:
-
Glomerular filtration rate
- mAb:
-
Monoclonal antibody
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus type 2
- VOC:
-
Variant of concern
References
Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–37. https://doi.org/10.1111/ajt.16369.
Koczulla RA, Sczepanski B, Koteczki A, et al. SARS-CoV-2 infection in two patients following recent lung transplantation. Am J Transplant. 2020;20:2928–32. https://doi.org/10.1111/ajt.15998.
Aversa M, Benvenuto L, Anderson M, et al. COVID-19 in lung transplant recipients: a single center case series from New York City. Am J Transplant. 2020;20:3072–80. https://doi.org/10.1111/ajt.16241.
Kamp JC, Hinrichs JB, Fuge J, Ewen R, Gottlieb J. COVID-19 in lung transplant recipients—risk prediction and outcomes. PLoS One. 2021;16: e0257807. https://doi.org/10.1371/journal.pone.0257807.
Heldman MR, Kates OS, Safa K, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic. Am J Transplant. 2022;22:279–88. https://doi.org/10.1111/ajt.16840.
Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation. 2021;105:e263–4. https://doi.org/10.1097/TP.0000000000003908.
Gottlieb J, Kolditz M, Gade N, Welte T, Kneidinger N. Benefit of monoclonal antibodies in early treatment of COVID-19 after lung transplantation—a retrospective analysis in two centers. Eur Respir J. 2022. https://doi.org/10.1183/13993003.00124-2022.
Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022. https://doi.org/10.1126/science.abn4947.
Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185:447-456.e11. https://doi.org/10.1016/j.cell.2021.12.032.
Hui KPY, Ho JCW, Cheung MC, et al. Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–20. https://doi.org/10.1038/s41586-022-04479-6.
Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–46. https://doi.org/10.1016/S0140-6736(22)00017-4.
Fall A, Eldesouki RE, Sachithanandham J, et al. A quick displacement of the SARS-CoV-2 variant delta with omicron: unprecedented spike in COVID-19 cases associated with fewer admissions and comparable upper respiratory viral loads. medRxiv. 2022. https://doi.org/10.1101/2022.01.26.22269927
Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–50. https://doi.org/10.1056/NEJMoa2107934.
Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–46. https://doi.org/10.1001/jama.2022.2832.
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. https://doi.org/10.1056/NEJMoa2116044
Wratil PR, Stern M, Priller A, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. 2022;28(3):496–503. https://doi.org/10.1038/s41591-022-01715-4.
Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment—a consensus report from the pulmonary council of the ISHLT. J Heart Lung Transplant. 2019;38:493–503. https://doi.org/10.1016/j.healun.2019.03.009.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–42. https://doi.org/10.1001/jama.2020.2648.
Bouzid D, Visseaux B, Kassasseya C, et al. Comparison of patients infected with delta versus omicron COVID-19 variants presenting to paris emergency departments: a retrospective cohort study. Ann Intern Med. 2022. https://doi.org/10.7326/M22-0308.
Maslo C, Friedland R, Toubkin M, et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. 2022;327:583–4. https://doi.org/10.1001/jama.2021.24868.
Havlin J, Skotnicova A, Dvorackova E, et al. Impaired humoral response to third dose of BNT162b2 mRNA COVID-19 vaccine despite detectable spike protein-specific T cells in lung transplant recipients. Transplantation. 2022;106:e183–4. https://doi.org/10.1097/TP.0000000000004021.
Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754–8. https://doi.org/10.1016/j.healun.2021.05.004.
Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386:305–15. https://doi.org/10.1056/NEJMoa2116846.
Salerno DM, Jennings DL, Lange NW, Kovac DB, Shertel T, Chen JK, Hedvat J, Scheffert J, Brown RS Jr, Pereira MR. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022. https://doi.org/10.1111/ajt.17027.
Gleeson S, Martin P, Thomson T, et al. Kidney transplant recipients and omicron: outcomes, effect of vaccines and the efficacy and safety of novel treatments. medRxiv. https://doi.org/10.1101/2022.05.03.22274524
Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–6. https://doi.org/10.1038/s41586-022-04594-4.
Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med. 2022;386:1475–1477. https://doi.org/10.1056/NEJMc2201933
Uraki R, Kiso M, Iida S, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2. Nature. 2022. https://doi.org/10.1038/s41586-022-04856-1
Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105:1365–71. https://doi.org/10.1097/TP.0000000000003670.
Funding
Open Access funding enabled and organized by Projekt DEAL. Not applicable.
Author information
Authors and Affiliations
Contributions
NK and JG designed and directed the project. All authors contributed to the data collection. NK and JG performed the analysis and drafted the manuscript. All authors were involved in interpretation of results reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Ethical approval
The study was approved by the central institutional ethics committee (LMU Munich, Germany; project number 22-0078).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kneidinger, N., Hecker, M., Bessa, V. et al. Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study. Infection 51, 749–757 (2023). https://doi.org/10.1007/s15010-022-01914-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01914-8