Abstract
Purpose
Early clinical failure criteria (ECFC) were recently introduced to predict unfavorable outcomes in patients with Gram-negative bloodstream infections (BSI). ECFC include hypotension, tachycardia, tachypnea or mechanical ventilation, altered mental status, and leukocytosis evaluated at 72–96 h after BSI. The aim of this retrospective cohort study was to assess performance of ECFC in predicting 28-day mortality in Enterococcus species BSI.
Methods
Hospitalized adults with Enterococcus species BSI at Prisma Health hospitals from 1 January 2015 to 31 July 2018 were identified. Multivariate logistic regression was used to determine the association between ECFC and 28-day mortality. Area under the receiver operating characteristic (AUROC) curve was used to measure model discrimination.
Results
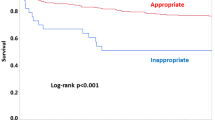
Among 157 patients, 28 (18%) died within 28 days of BSI. After adjustments in multivariate model, the risk of 28-day mortality increased in the presence of each additional ECFC (OR 1.6, 95% CI 1.2–2.3, p = 0.005). Infective endocarditis (OR 3.9, 95% CI 1.4–10.7, p = 0.01) was independently associated with 28-day mortality. AUROC curve of ECFC model in predicting 28-day mortality was 0.74 with ECFC of 2 identified as the best breakpoint. Mortality was 8% in patients with ECFC < 2 compared to 33% in those with ECFC ≥ 2 (p < 0.001).
Conclusion
ECFC had good discrimination in predicting 28-day mortality in patients with Enterococcus species BSI. These criteria may have utility in future clinical investigations.

Similar content being viewed by others
Availability of data and material
The authors declare that all data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Pinholt M, Ostergarrd C, Apri M, et al. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006–2009: a population-based cohort study. Clin Microbiol Infect. 2014;20:145–51.
DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect. 2005;191:588–95.
Billington EO, Phang SH, Gregson DB, et al. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis. 2014;26:76–82.
Vergis EN, Hayden MK, Chow JW, et al. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. Ann Intern Med. 2001;135:484–92.
McBride SJ, Upton A, Roberts SA. Clinical characteristics and outcomes of patients with vancomycin-susceptible Enterococcus faecalis and Enterococcus faecium bacteraemia—a five-year retrospective review. Eur J Clin Microbiol Infect Dis. 2010;29:107–14.
Lodise TP, McKinnon PS, Tam VH, et al. Clinical outcomes for patients with bacteremia caused by vancomycin-resistant enterococcus in a level 1 trauma center. Clin Infect Dis. 2002;34:922–9.
Oliver CN, Blake RK, Steed LL, et al. Risk of vancomycin-resistant enterococcus (VRE) bloodstream infection among patients colonized with VRE. Infect Control Hosp Epidemiol. 2008;29:404–9.
Lagnf AM, Zasowski EJ, Claeys KC, et al. Comparison of clinical outcomes and risk factors in polymicrobial versus monomicrobial enterococcal bloodstream infections. Am J Infect Control. 2016;44:917–21.
Rac H, Gould AP, Bookstaver PB, et al. Evaluation of early clinical failure criteria for gram-negative bloodstream infections. Clin Microbiol Infect. 2020;26:73–7.
Laupland KA, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2017;27:647–64.
Friedman ND, Kaye KS, Stout JE, et al. Healthcare–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32.
Quinn NJ, Sebaaly JC, Patel BA, et al. Effectiveness of oral antibiotics for definitive therapy of non-Staphylococcal Gram-positive bacterial bloodstream infections. Ther Adv Infectious Dis. 2019;6:1–12.
Hale AJ, Snyder GM, Ahern JW, et al. When are oral antibiotics a safe and effective choice for bacterial bloodstream infections? An evidence-based narrative review. J Hosp Med. 2018;13:328–35.
Bhavnani SM, Drake JA, Forrest A, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn Microbiol Infect Dis. 2000;36:145–58.
Herrmann L, Kimmig A, Rödel J, et al. Early treatment outcomes for bloodstream infections caused by potential AmpC beta-lactamase-producing Enterobacterales with focus on piperacillin/tazobactam: a retrospective cohort study. Antibiotics (Basel). 2021;10:665. https://doi.org/10.3390/antibiotics10060665.
Acknowledgements
The authors thank Prisma Health Antimicrobial Stewardship and Support Team in South Carolina, USA for their help in facilitating the conduct of this study. The preliminary results of this study were presented in part at IDWeek annual meeting on October 5, 2019 in Washington DC, USA.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Study conception and design, CEP, PBB, CC, JAJ, JK, HRW, MNA-H. Material preparation, CEP, JK. Data collection, CEP, AB. Analysis, CEP, PBB, MNA-H. Manuscript writing-first draft, CEP, PBB, MNA-H. Manuscript writing-reviewing and editing, CEP, PBB, AB, CC, JAJ, JK, HRW, MNA-H.
Corresponding author
Ethics declarations
Conflict of interest
P.B.B: research advisory board for Kedrion Biopharma. J.A.J: Merck & Co., Inc; VaxArt. C.E.P, C.C., A.B., H.R.W., J.K.: no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Powers, C.E., Bookstaver, P.B., Caulder, C. et al. Evaluation of early clinical failure criteria in Enterococcus species bloodstream infection. Infection 50, 873–877 (2022). https://doi.org/10.1007/s15010-022-01754-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01754-6