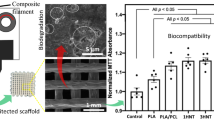
Abstract
Background:
Poly (lactic acid) (PLA) is a biodegradable polyester that has been exploited for a variety of biomedical applications, including tissue engineering. The incorporation of β-tricalcium phosphate (TCP) into PLA has imparted bioactivity to the polymeric matrix.
Methods:
We have modified a 90%PLA-10%TCP composite with SiO2 and MgO (1, 5 and 10 wt%), separately, to further enhance the material bioactivity. Filaments were prepared by extrusion, and scaffolds were fabricated using 3D printing technology associated with fused filament fabrication.
Results:
The PLA-TCP-SiO2 composites presented similar structural, thermal, and rheological properties to control PLA and PLA-TCP. In contrast, the PLA-TCP-MgO composites displayed absence of crystallinity, lower polymeric molecular weight, accelerated degradation ratio, and decreased viscosity within the 3D printing shear rate range. SiO2 and MgO particles were homogeneously dispersed within the PLA and their incorporation increased the roughness and protein adsorption of the scaffold, compared to a PLA-TCP scaffold. This favorable surface modification promoted cell proliferation, suggesting that SiO2 and MgO may have potential for enhancing the bio-integration of scaffolds in tissue engineering applications. However, high loads of MgO accelerated the polymeric degradation, leading to an acid environment that imparted the composite biocompatibility. The presence of SiO2 stimulated mesenchymal stem cells differentiation towards osteoblast; enhancing extracellular matrix mineralization, alkaline phosphatase (ALP) activity, and bone-related genes expression.
Conclusion:
The PLA-10%TCP-10%SiO2 composite presented the most promising results, especially for bone tissue regeneration, due to its intense osteogenic behavior. PLA-10%TCP-10%SiO2 could be used as an alternative implant for bone tissue engineering application.









Similar content being viewed by others
Data availability statement
The data presented in this study are available on request from all the authors.
References
Chou YC, Lee D, Chang TM, Hsu YH, Yu YH, Liu SJ, et al. Development of a three-dimensional (3D) printed biodegradable cage to convert morselized corticocancellous bone chips into a structured cortical bone graft. Int J Mol Sci. 2016;17:e595.
Radwan-Pragłowska J, Janus Ł, Piątkowski M, Bogdał D, Matýsek D. Hybrid bilayer PLA/chitosan nanofibrous scaffolds doped with ZnO, Fe3O4, and Au nanoparticles with bioactive properties for skin tissue engineering. Polymers. 2020;12:e159.
Haaparanta AM, Järvinen E, Cengiz IF, Ellä V, Kokkonen HT, Kiviranta I, et al. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2014;25:1129–36.
Nakayama KH, Shayan M, Huang NF. Engineering biomimetic materials for skeletal muscle repair and regeneration. Adv Healthc Mater. 2019;8:e1801168.
Araque-Monrós MC, García-Cruz DM, Escobar-Ivirico JL, Gil-Santos L, Monleón-Pradas M, Más-Estellés J. Regenerative and resorbable PLA/HA hybrid construct for tendon/ligament tissue engineering. Ann Biomed Eng. 2020;48:757–67.
Naseri-Nosar M, Salehi M, Hojjati-Emami S. Cellulose acetate/poly lactic acid coaxial wet-electrospun scaffold containing citalopram-loaded gelatin nanocarriers for neural tissue engineering applications. Int J Biol Macromol. 2017;103:701–8.
Gugutkov D, Gustavsson J, Cantini M, Salmeron-Sánchez M, Altankov G. Electrospun fibrinogen-PLA nanofibres for vascular tissue engineering. J Tissue Eng Regen Med. 2017;11:2774–84.
Kang Y, Wang C, Qiao Y, Gu J, Zhang H, Peijs T, et al. Tissue-engineered trachea consisting of electrospun patterned Sc-PLA/GO- G-IL fibrous membranes with antibacterial property and 3D-printed skeletons with elasticity. Biomacromol. 2019;20:1765–76.
Zhang H, Mao X, Zhao D, Jiang W, Du Z, Li Q, et al. Three dimensional printed polylactic acid-hydroxyapatite composite scaffolds for prefabricating vascularized tissue engineered bone: an in vivo bioreactor model. Sci Rep. 2017;7:e15255.
Orafa Z, Irani S, Zamanian A, Bakhshi H, Nikukar H, Ghalandari B. Coating of laponite on PLA nanofibrous for bone tissue engineering application. Macromol Res. 2021;29:191–8.
Backes EH, LdeN P, Selistre-de-Araujo HS, Costa LC, Passador FR, Pessan LA. Development and characterization of printable PLA/Β-TCP bioactive composites for bone tissue applications. J Appl Polym Sci. 2020;138:e49759.
Backes EH, LdeN P, Beatrice CAG, Costa LC, Passador FR, Pessan LA. Fabrication of biocompatible composites of poly(lactic acid)/hydroxyapatite envisioning medical applications. Polym Eng Sci. 2020;60:636–44.
Backes EH, Fernandes EM, Diogo GS, Marques CF, Silva TH, Costa LC, et al. Engineering 3D printed bioactive composite scaffolds based on the combination of aliphatic polyester and calcium phosphates for bone tissue regeneration. Mater Sci Eng C. 2021;122:e111928.
Wang W, Zhang B, Li M, Li J, Zhang C, Han Y, et al. 3D Printing of PLA/N-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos B Eng. 2021;224:e109192.
Hickey DJ, Ercan B, Sun L, Webster TJ. Adding MgO nanoparticles to hydroxyapatite–PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015;14:175–84.
Wang S, Wang X, Draenert FG, Albert O, Schröder HC, Mailänder V, et al. Bioactive and biodegradable silica biomaterial for bone regeneration. Bone. 2014;67:292–304.
Huang B. Carbon nanotubes and their polymeric composites: the applications in tissue engineering. Biomanuf Rev. 2020;5:e3.
Sergi R, Bellucci D, Cannillo V. A review of bioactive glass/natural polymer composites: state of the art. Materials. 2020;13:e5560.
Zhai X, Ma Y, Hou C, Gao F, Zhang Y, Ruan C, et al. 3D-printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater Sci Eng. 2017;3:1109–18.
Ren X, Zhao M, Lash B, Martino MM, Julier Z. Growth factor engineering strategies for regenerative medicine applications. Front Bioeng Biotechnol. 2020;7:e469.
Castelletto V, Gouveia RJ, Connon CJ, Hamley IW. Self-assembly and bioactivity of a polymer/peptide conjugate containing the RGD cell adhesion motif and PEG. Eur Polym J. 2013;49:2961–7.
Nie X, Sun X, Wang C, Yang J. Effect of magnesium ions/type I collagen promote the biological behavior of osteoblasts and its mechanism. Regen Biomater. 2020;7:53–61.
Zhao Y, Liu B, You C, Chen M. Effects of MgO whiskers on mechanical properties and crystallization behavior of PLLA/MgO composites. Mater Des. 2016;89:573–81.
Roh HS, Lee CM, Hwang YH, Kook MS, Yang SW, Lee D, et al. Addition of MgO nanoparticles and plasma surface treatment of three-dimensional printed polycaprolactone/hydroxyapatite scaffolds for improving bone regeneration. Mater Sci Eng C. 2017;74:525–35.
Pascual-González C, Thompson C, Jdela V, Churruca NB, Fernández-Blázquez JP, Lizarralde I, et al. Processing and properties of PLA/Mg filaments for 3D printing of scaffolds for biomedical applications. Rapid Prototyp J. 2021;28:884–94.
Swetha S, Balagangadharan K, Lavanya K, Selvamurugan N. Three-dimensional-poly(lactic acid) scaffolds coated with gelatin/magnesium-doped nano-hydroxyapatite for bone tissue engineering. Biotechnol J. 2021;16:e2100282.
Brown A, Zaky S, RaySfeir HC Jr. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015;11:543–53.
Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74.
Tang L, Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano Today. 2013;8:290–312.
Valliant EM, Romer F, Wang D, McPhail DS, Smith ME, Hanna JV, et al. Bioactivity in silica/poly(c-glutamic acid) sol–gel hybrids through calcium chelation. Acta Biomater. 2013;9:7662–71.
Catauro M, Bollino F, Papale F. Surface modifications of titanium implants by coating with bioactive and biocompatible poly (ε-caprolactone)/SiO2 hybrids synthesized via sol-gel. Arab J Chem. 2018;11:1126–33.
Sachot N, Castano O, Mateos-Timoneda MA, Engel E, Planell JA. Hierarchically engineered fibrous scaffolds for bone regeneration. J R Soc Interface. 2013;10:e20130684.
Yang X, Li Y, Liu X, Huang Q, Zhang R, Feng Q. Incorporation of silica nanoparticles to PLGA electrospun fibers for osteogenic differentiation of human osteoblast-like cells. Regen Biomater. 2018;5:229–38.
Shi M, Zhou Y, Shao J, Chen Z, Song B, Chang J, et al. Stimulation of osteogenesis and angiogenesis of HBMSCS by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015;21:178–89.
Yang X, Li Y, Liu X, Huang Q, He W, Zhang R, et al. The stimulatory effect of silica nanoparticles on osteogenic differentiation of human mesenchymal stem cells. Biomed Mater. 2017;12:e015001.
Ha S, Weitzmann MN, Beck GR. Bioactive silica nanoparticles promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano. 2014;8:5898–910.
Mladenovic Z, Johansson A, Willman B, Shahabi K, Björn E, Ransjö M. Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater. 2014;10:406–18.
Chen X, Chen G, Wang G, Zhu P, Gao C. Recent progress on 3D-printed polylactic acid and its applications in bone repair. Adv Eng Mater. 2020;22:e1901065.
Attaran M. The rise of 3-D printing: the advantages of additive manufacturing over traditional manufacturing. Bus Horiz. 2017;60:677–88.
Santos LG, Costa LC, Pessan LA. Development of biodegradable PLA/PBT nanoblends. J Appl Polym Sci. 2017;135:e45951.
Li Y, Li Q, Yang G, Ming R, Yu M, Zhang H, et al. Evaluation of thermal resistance and mechanical properties of injected molded stereocomplex of poly(L-lactic acid) and poly(D-lactic acid) with various molecular weights. Adv Polym Technol. 2018;37:1674–81.
McIlroy C, Olmsted PD. Disentanglement effects on welding behaviour of polymer melts during the fused-filament-fabrication method for additive manufacturing. Polymer. 2017;123:376–91.
Costa SF, Duarte FM, Covas JA. Estimation of filament temperature and adhesion development in fused deposition techniques. J Mater Process Technol. 2017;245:167–79.
Sanchez LC, Beatrice CAG, Lotti C, Marini J, Bettini SHP, Costa LC. Rheological approach for an additive manufacturing printer based on material extrusion. J Adv Manuf Technol. 2019;105:2403–14.
Backes EH, Beatrice CAG, Shimomura KMB, Harb SV, Pachane BC, Selestre-de-Araujo HS, et al. Development of poly(ε-polycaprolactone)/hydroxyapatite composites for bone tissue regeneration. J Mater Res. 2021;36:3050–62.
Beatrice CAG, Shimomura KMB, Backes EH, Harb SV, Costa LC, Passador FR, et al. Engineering printable composites of poly(ɛ-polycaprolactone)/β-tricalcium phosphate for biomedical applications. Polym Compos. 2021;42:1198–213.
Nofar M, Salehiyan R, Ray SS. Rheology of poly (lactic acid)-based systems. Polym Rev. 2019;59:465–509.
Denry I, Kuhn LT. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent Mater. 2016;32:43–53.
Kumar A, Mandal S, Barui S, Vasireddi R, Gbureck U, Gelinsky M, et al. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: processing related challenges and property assessment. Mater Sci Eng R Rep. 2016;103:1–39.
Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010;31:1465–85.
Rogowska-Tylman J, Locs J, Salma I, Woźniak B, Pilmane M, Zalite V, et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Mater Sci Eng C. 2019;99:669–84.
Backes EH, Harb SV, Beatrice CAG, Shimomura KMB, Passador FR, Costa LC, et al. Polycaprolactone usage in additive manufacturing strategies for tissue engineering applications: a review. J Biomed Mater Res. 2022;110:1479–503.
Zheng Y, Han Q, Li D, Sheng F, Song Z, Wang J. Promotion of tendon growth into implant through pore-size design of a Ti-6Al-4 V porous scaffold prepared by 3D printing. Mater Des. 2021;197:e109219.
Zhang H, Pei Z, Wang C, Li M, Zhang H, Qu J. Electrohydrodynamic 3D printing scaffolds for repair of achilles tendon defect in rats. Tissue Eng Part A. 2021;27:1343–54.
Kosorn W, Sakulsumbat M, Uppanan P, Kaewkong P, Chantaweroad S, Jitsaard J, et al. PCL/PHBV blended three dimensional scaffolds fabricated by fused deposition modeling and responses of chondrocytes to the scaffolds. J Biomed Mater Res Part B. 2017;105:1141–50.
Guimarães CF, Gasperini L, Marques AP, Reis RL. The stiffness of living tissues and its implications for tissue engineering. Nat Rev Mater. 2020;5:351–70.
Hutmacher DW, Schantz JT, Lam CXF, Tan KC, Lim TC. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245–60.
Rosales-Leal JI, Rodríguez-Valverde MA, Mazzaglia G, Ramón-Torregrosa PJ, Díaz-Rodríguez L, García-Martínez O, et al. Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion. Colloid Surf A. 2010;365:222–9.
Bagherifard S, Hickey DJ, de Luca AC, Malheiro VN, Markaki AE, Guagliano M, et al. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials. 2015;73:185–97.
Harb SV, Bassous NJ, de Souza TAC, Trentin A, Pulcinelli SH, Santilli CV, et al. Hydroxyapatite and β-TCP modified PMMA-TiO2 and PMMA-ZrO2 coatings for bioactive corrosion protection of Ti6Al4V implants. Mater Sci Eng C. 2020;116:111149.
Harb SV, Uvida MC, Trentin A, Lobo AO, Webster TJ, Pulcinelli SH, et al. PMMA-Silica nanocomposite coating: effective corrosion protection and biocompatibility for a Ti6Al4V alloy. Mater Sci Eng C. 2020;110:110713.
Shasteen C, Choy YB. Controlling degradation rate of poly(lactic acid) for its biomedical applications. Biomed Eng Lett. 2011;1:e163.
Xiao L, Wang B, Yang G, Gauthier M. Poly(lactic acid)-based biomaterials: synthesis, modification and applications. biomedical science, engineering and technology, in: D.N. Ghista (Ed.), Biomedical Science, Engineering and Technology, Intechopen, London, 2012, pp. 247–282.
Samadian H, Farzamfar S, Vaez A, Ehterami A, Bit A, Alam M, et al. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and taurine for bone regeneration. Sci Rep. 2020;10:e13366.
Murariu M, Dubois P. PLA composites: from production to properties. Adv Drug Deliv Rev. 2016;107:17–46.
Siqueira IAWB, Amaral SS, de Moura NK, Machado JPB, Backes EH, Passador FR, et al. In vitro bioactivity and biological assays of porous membranes of the poly(lactic acid) containing calcium silicate fibers. Polym Bull. 2020;77:5357–71.
Chen X, Gao C, Jiang J, Wu Y, Zhu P, Chen G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed Mater. 2019;14:e065003.
Marra A, Cimmino S, Silvestre C. Effect of TiO2 and ZnO on PLA degradation in various media. Adv Mater Sci. 2017;2:1–8.
Narayanan G, Vernekar VN, Kuyinu EL, Laurencin CT. Poly(lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev. 2016;107:247–76.
Shuai C, Li Y, Feng P, Guo W, Yang W, Peng S. Positive feedback effects of mg on the hydrolysis of poly-l-lactic acid (PLLA): promoted degradation of PLLA scaffolds. Polym Test. 2018;68:27–33.
Bakhshi R, Mohammadi-Zerankeshi M, Mehrabi-Dehdezi M, Alizadeh R, Labbaf S, Abachi P. Additive manufacturing of PLA-Mg composite scaffolds for hard tissue engineering applications. J Mech Behav Biomed Mater. 2023;138:105655.
Nilawar S, Chatterjee K. Surface decoration of redox-modulating nanoceria on 3D-printed tissue scaffolds promotes stem cell osteogenesis and attenuates bacterial colonization. Biomacromol. 2022;23:226–39.
Dong L, Wang SJ, Zhao XR, Zhu YF, Yu JK. 3D- printed poly(ε-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering. Sci Rep. 2017;7:13412.
Acknowledgements
The authors would like to thank Dr. Craig Neal for the scientific contribution, and to the Laboratory of Structural Characterization (LCE/DEMa/UFSCar) for SEM-EDS and AFM facilities. This study was financed by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant numbers 2021/11538-8, 2018/26060-3, 2019/27415-2, 2017/09609-9, and 2017/11366-7], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 [grant numbers 88887.485864/2020-00 and 88887.512147/2020-00), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number 306835/2017-7], and the UCF Preeminent Postdoctoral Program (P3) [E.K. grant].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harb, S.V., Kolanthai, E., Backes, E.H. et al. Effect of Silicon Dioxide and Magnesium Oxide on the Printability, Degradability, Mechanical Strength and Bioactivity of 3D Printed Poly (Lactic Acid)-Tricalcium Phosphate Composite Scaffolds. Tissue Eng Regen Med 21, 223–242 (2024). https://doi.org/10.1007/s13770-023-00584-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00584-3