Abstract
Background:
Mesenchymal stem cells (MSCs) are considered a potential tool for regenerating damaged tissues due to their great multipotency into various cell types. Here, we attempted to find the appropriate conditions for neuronal differentiation of tonsil-derived MSCs (TMSCs) and expand the potential application of TMSCs for treating neurological diseases.
Methods:
The TMSCs were differentiated in DMEM/F-12 (Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12) supplemented with various neurotrophic factors for 7–28 days to determine the optimal neuronal differentiation condition for the TMSCs. The morphologies as well as the levels of the neural markers and neurotransmitters were assessed to determine neuronal differentiation potentials and the neuronal lineages of the differentiated TMSCs.
Results:
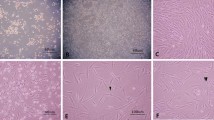
Our initial study demonstrated that DMEM/F12 supplemented with 50 ng/mL basic fibroblast growth factor with 10 μM forskolin was the optimal condition for neuronal differentiation for the TMSCs. TMSCs had higher protein expression of neuronal markers, including neuron-specific enolase (NSE), GAP43, postsynaptic density protein 95 (PSD95), and synaptosomal-associated protein of 25 kDa (SNAP25) compared to the undifferentiated TMSCs. Immunofluorescence staining also validated the increased mature neuron markers, NeuN and synaptophysin, in the differentiated TMSCs. The expression of glial fibrillar acidic protein and ionized calcium-binding adaptor molecule 1 the markers of astrocytes and microglia, were also slightly increased. Additionally, the differentiated TMSCs released a significantly higher level of acetylcholine, the cholinergic neurotransmitter, as analyzed by the liquid chromatography-tandem mass spectrometry and showed an enhanced choline acetyltransferase immunoreactivity compared to the undifferentiated cells.
Conclusion:
Our study suggests that the optimized condition favors the TMSCs to differentiate into cholinergic neuron-like phenotype, which could be used as a possible therapeutic tool in treating certain neurological disorders such as Alzheimer’s disease.




Similar content being viewed by others
References
GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:459–80.
Grade S, Götz M. Neuronal replacement therapy: previous achievements and challenges ahead. NPJ Regen Med. 2017;2:29.
Jeong HJ, Jimenez Z, Mukhambetiyar K, Seo M, Choi JW, Park TE. Engineering human brain organoids: from basic research to tissue regeneration. Tissue Eng Regen Med. 2020;17:747–57.
Bian J, Zheng J, Li S, Luo L, Ding F. Sequential differentiation of embryonic stem cells into neural epithelial-like stem cells and oligodendrocyte progenitor cells. PLoS One. 2016;11:e0155227.
D’Aiuto L, Zhi Y, Kumar Das D, Wilcox MR, Johnson JW, McClain L, et al. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. Organogenesis. 2014;10:365–77.
King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther. 2014;5:85.
Medvedev SP, Shevchenko AI, Zakian SM. Induced pluripotent stem cells: problems and advantages when applying them in regenerative medicine. Acta Naturae. 2010;2:18–28.
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY, Jo I, et al. Tonsil-derived mesenchymal stromal cells: evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy. 2012;14:1193–202.
Van Nguyen TT, Vu NB, Van Pham P. Mesenchymal stem cell transplantation for ischemic diseases: mechanisms and challenges. Tissue Eng Regen Med. 2021;18:587–611.
Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35: e00191.
Venkatesh K, Sen D. Mesenchymal stem cells as a source of dopaminergic neurons: a potential cell based therapy for parkinson’s disease. Curr Stem Cell Res Ther. 2017;12:326–47.
Urrutia DN, Caviedes P, Mardones R, Minguell JJ, Vega-Letter AM, Jofre CM. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: an approach for their use in neural regeneration therapies. PLoS One. 2019;14:0213032.
Oh SY, Choi YM, Kim HY, Park YS, Jung SC, Park JW, et al. Application of tonsil-derived mesenchymal stem cells in tissue regeneration: concise review. Stem Cells. 2019;37:1252–60.
Arad M, Brown RA, Khatri R, Taylor RJ, Zalzman M. Direct differentiation of tonsillar biopsy-derived stem cells to the neuronal lineage. Cell Mol Biol Lett. 2021;26:38.
Jung N, Park S, Choi Y, Park JW, Hong YB, Park HH, et al. Tonsil-derived mesenchymal stem cells differentiate into a schwann cell phenotype and promote peripheral nerve regeneration. Int J Mol Sci. 2016;17:1867.
Patel M, Moon HJ, Jung BK, Jeong B. Microsphere-incorporated hybrid thermogel for neuronal differentiation of tonsil derived mesenchymal stem cells. Adv Healthc Mater. 2015;4:1565–74.
Li VC, Kirschner MW. Molecular ties between the cell cycle and differentiation in embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:9503–8.
Enders M, Heider T, Ludwig A, Kuerten S. Strategies for neuroprotection in multiple sclerosis and the role of calcium. Int J Mol Sci. 2020;21:1663.
Lim JY, Park SI, Kim SM, Jun JA, Oh JH, Ryu CH, et al. Neural differentiation of brain-derived neurotrophic factor-expressing human umbilical cord blood-derived mesenchymal stem cells in culture via TrkB-mediated ERK and β-catenin phosphorylation and following transplantation into the developing brain. Cell Transplant. 2011;20:1855–66.
Zeng S, Zhao X, Zhang L, Pathak JL, Huang W, Li Y, et al. Effect of ciliary neurotrophic factor on neural differentiation of stem cells of human exfoliated deciduous teeth. J Biol Eng. 2020;14:29.
Kosaka N, Kodama M, Sasaki H, Yamamoto Y, Takeshita F, Takahama Y, et al. FGF-4 regulates neural progenitor cell proliferation and neuronal differentiation. FASEB J. 2006;20:1484–5.
Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70:271–88.
Inazawa T, Okamura Y, Takahashi K. Basic fibroblast growth factor induction of neuronal ion channel expression in ascidian ectodermal blastomeres. J Physiol. 1998;511:347–59.
Souttou B, Carvalho NBD, Raulais D, Vigny M. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J Biol Chem. 2001;276:9526–31.
Galy A, Néron B, Planque N, Saule S, Eychène A. Activated MAPK/ERK kinase (MEK-1) induces transdifferentiation of pigmented epithelium into neural retina. Dev Biol. 2002;248:251–64.
Xu S, De Veirman K, Evans H, Santini GC, Vande Broek I, Leleu X, et al. Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo. Acta Pharmacol Sin. 2013;34:699–709.
Askvig JM, Watt JA. The MAPK and PI3K pathways mediate CNTF-induced neuronal survival and process outgrowth in hypothalamic organotypic cultures. J Cell Commun Signal. 2015;9:217–31.
Lu T, Yang C, Sun H, Lv J, Zhang F, Dong XJ. FGF4 and HGF promote differentiation of mouse bone marrow mesenchymal stem cells into hepatocytes via the MAPK pathway. Genet Mol Res. 2014;13:415–24.
Revest JM, Le Roux A, Roullot-Lacarrière V, Kaouane N, Vallée M, Kasanetz F, et al. BDNF-TrkB signaling through Erk1/2MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Mol Psychiatry. 2014;19:1001–9.
Lee S, Choi K, Ahn H, Song K, Choe J, Lee I. TuJ1 (class III β-tubulin) expression suggests dynamic redistribution of follicular dendritic cells in lymphoid tissue. Eur J Cell Biol. 2005;84:453–9.
Cho KA, Kim JY, Kim HS, Ryu KH, Woo SY. Tonsil-derived mesenchymal progenitor cells acquire a follicular dendritic cell phenotype under cytokine stimulation. Cytokine. 2012;59:211–4.
Andreska T, Aufmkolk S, Sauer M, Blum R. High abundance of BDNF within glutamatergic presynapses of cultured hippocampal neurons. Front Cell Neurosci. 2014;8:107.
Rauti R, Cellot G, D’Andrea P, Colliva A, Scaini D, Tongiorgi E, et al. BDNF impact on synaptic dynamics: extra or intracellular long-term release differently regulates cultured hippocampal synapses. Mol Brain. 2020;13:43.
Kim MH, Park SR, Choi BH. Comparative analysis of the expression of chondroitin sulfate subtypes and their inhibitory effect on axonal growth in the embryonic, adult, and injured rat brains. Tissue Eng Regen Med. 2021;18:165–78.
Perkins LA, Cain LD. Basic fibroblast growth factor (bFGF) increases the survival of embryonic and postnatal basal forebrain cholinergic neurons in primary culture. Int J Dev Neurosci. 1995;13:51–61.
Calza L, Giuliani A, Fernandez M, Pirondi S, D'Intino G, Aloe L, et al. Neural stem cells and cholinergic neurons: regulation by immunolesion and treatment with mitogens, retinoic acid, and nerve growth factor. Proc Natl Acad Sci U S A. 2003;100:7325–30.
Kang YH, Shivakumar SB, Son YB, Bharti D, Jang SJ, Heo KS, et al. Comparative analysis of three different protocols for cholinergic neuron differentiation in vitro using mesenchymal stem cells from human dental pulp. Anim Cells Syst (Seoul). 2019;23:275–87.
Choi DH, Lee KE, Oh SY, Lee SM, Jo BS, Lee JY, et al. Tonsil-derived mesenchymal stem cells incorporated in reactive oxygen species-releasing hydrogel promote bone formation by increasing the translocation of cell surface GRP78. Biomaterials. 2021;278:121156.
Rasband MN. Glial contributions to neural function and disease. Mol Cell Proteomics. 2016;15:355–61.
Acknowledgements
This work was financially supported by the grant of the National Research Foundation of Korea funded by the Korean Government (2019R1A2C1086767) and Creative and Exploratory Research (2021R1I1A1A01046931). The research was also supported by the RP-Grant 2021 from Ewha Womans University. We also thank NeuroVIS (Hwaseoung-si, Gyeonggi-do, Republic of Korea) for LC-MS/MS analysis of the neurotransmitter concentrations in the culture media.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) of the Ewha Womans University Medical Center institutional review board (ECT-11-53-02). Informed written consent forms were collected from all patients and/or their legal parents/guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, JH., Oh, SY. & Jo, S.A. Basic Fibroblast Growth Factor Induces Cholinergic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. Tissue Eng Regen Med 19, 1063–1075 (2022). https://doi.org/10.1007/s13770-022-00474-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00474-0