Abstract
Background:
This study aimed to observe the cartilaginous matrix production in SRY (sex determining region Y)-box 9 (SOX9)- and/or telomerase reverse transcriptase (TERT)-transfected chondrocytes from monolayer to three-dimensional (3D) culture.
Methods:
The genes were transferred into chondrocytes at passage-1 (P1) via lipofection. The post-transfected chondrocytes (SOX9-, TERT- and SOX9/TERT) were analysed at P1, P2 and P3. The non-transfected group was used as control. The 3D culture was established using the chondrocytes seeded in a disc-shaped PLGA/fibrin and PLGA scaffolds. The resulting 3D “cells-scaffolds” constructs were analysed at week-1, -2 and -3. The histoarchitecture was evaluated using haematoxylin and eosin, alcian blue and safranin o stains. The quantitative sulphated glycosaminoglycan (sGAG) content was measured using biochemical assay. The cartilage-specific markers expression were analysed via real-time polymerase chain reaction.
Results:
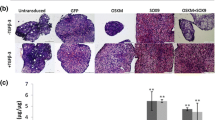
All monolayer cultured chondrocytes showed flattened, fibroblast-like appearance throughout passages. Proteoglycan and sGAG were not detected at the pericellular matrix region of the chondrocytes. The sGAG content assay indicated the matrix production depletion in the culture. The cartilage-specific markers, COL2A1 and ACAN, were downregulated. However, the dedifferentiation marker, COL1A1 was upregulated. In 3D “cells-scaffolds” constructs, regardless of transfection groups, chondrocytes seeded in PLGA/fibrin showed a more uniform distribution and produced denser matrix than the PLGA group especially at week-3. Both sGAG and proteoglycan were clearly visualised in the constructs, supported by the increment of sGAG content, quantitatively. Both COL2A1 and ACAN were upregulated in SOX9/TERT-PLGA and SOX9/TERT-PLGA/fibrin respectively. While, COL1A1 was downregulated in SOX9/TERT-PLGA.
Conclusion:
These findings indicated that the SOX9/TERT-transfected chondrocytes incorporation into 3D scaffolds facilitates the cartilage regeneration which is viable structurally and functionally.






Similar content being viewed by others
References
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6.
Abdul Rahman R, Mohamad Sukri N, Md Nazir N, Ahmad Radzi MA, Zulkifly AH, Che Ahmad A, et al. The potential of 3-dimensional construct engineered from poly(lactic-co-glycolic acid)/fibrin hybrid scaffold seeded with bone marrow mesenchymal stem cells for in vitro cartilage tissue engineering. Tissue Cell. 2015;47:420–30.
Sha’ban M, Osman Cassim S, Mohd Yahya NH, Saim A, Hj Idrus R. Sox-9 transient transfection enhances chondrogenic expression of osteoarthritic human articular chondrocytes in vitro: preliminary analysis. Tissue Eng Regen Med. 2011;8:32–41.
Frisch J, Rey-Rico A, Venkatesan JK, Schmitt G, Madry H, Cucchiarini M. TGF-β gene transfer and overexpression via rAAV vectors stimulates chondrogenic events in human bone marrow aspirates. J Cell Mol Med. 2016;20:430–40.
Griffin DJ, Ortved KF, Nixon AJ, Bonassar LJ. Mechanical properties and structure-function relationships in articular cartilage repaired using IGF-I gene-enhanced chondrocytes. J Orthop Res. 2016;34:149–53.
Bleiziffer O, Eriksson E, Yao F, Horch RE, Kneser U. Gene transfer strategies in tissue engineering. J Cell Mol Med. 2007;11:206–23.
Sha’ban M, Saim A, Osman Cassim S, Chua KH, Nor Hussein F, Hj Idrus R. The re-expression of collagen type II, aggrecan and Sox9 in tissue-engineered human articular cartilage. Tissue Eng Regen Med. 2005;2:347–55.
O’Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011;13:3.
Nazir NM, Zulkifly AH, Khalid KA, Zainol I, Zamli Z, Shaban M. Effects of SRY (Sex Determining Region Y)-Box 9 (SOX9) and Telomerase Reverse Transcriptase (TERT) genes transfection in chondrocytes seeded on three-dimensional scaffolds: Gross observation and cell proliferation assay. Front Bioeng Biotechnol. 2016. https://doi.org/10.3389/conf.FBIOE.2016.02.00027.
Camarero-Espinosa S, Rothen-Rutishauser B, Weder C, Foster EJ. Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials. 2016;74:42–52.
Ghassemi T, Shahroodi A, Ebrahimzadeh MH, Mousavian A, Movaffagh J, Moradi A. Current concepts in scaffolding for bone tissue engineering. Arch Bone Jt Surg. 2018;6:90–9.
Cheng A, Schwartz Z, Kahn A, Li X, Shao Z, Sun M, et al. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng Part B Rev. 2019;25:14–29.
Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19:485–502.
Ko CY, Ku KL, Yang SR, Lin TY, Peng S, Peng YS, et al. In vitro and in vivo co-culture of chondrocytes and bone marrow stem cells in photocrosslinked PCL-PEG-PCL hydrogels enhances cartilage formation. J Tissue Eng Regen Med. 2016;10:E485–96.
Munirah S, Kim SH, Ruszymah BH, Khang G. The use of fibrin and poly(lactic-co-glycolic acid) hybrid scaffold for articular cartilage tissue engineering: an in vivo analysis. Eur Cells Mater. 2008;15:41–52.
Sha’ban M, Kim SH, Idrus RB, Khang G. Fibrin and poly(lactic-co-glycolic acid) hybrid scaffold promotes early chondrogenesis of articular chondrocytes: an in vitro study. J Orthop Surg Res. 2008;3:17.
Mohamad Sukri N, Ahmad Radzi MA, Abdul Rahman R, Zulkifly AH, Abdulahi Hashi A, Sha’ban M. Identifying the potential of transcription factor SOX9 gene transfer in chondrocytes differentiation and articular cartilage formation in vitro. J Teknol. 2015;77:107–13.
O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today (Kidlington). 2011;14:88–95.
Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17:S467–79.
Md Nazir N, Mohammad MY, Muhammad Radzi MAA, Hashim R, Mat Nawi NF, Zulkifly AH, et al. Identifying transfection efficiency and cartilaginous markers expression in chondrocytes overexpressed with SRY (Sex Determining Region Y)-Box 9 (SOX9) gene: a preliminary analysis in an in vitro model. In: Proceedings of the international conference on biotechnology engineering ICBioE ’16, vol. 1. 2016. p. 9106–9.
Karlsson M, Björnsson S. Quantitation of proteoglycans in biological fluids using Alcian blue. Methods Mol Biol. 2001;171:159–73.
Mohamad MY, Muhammad Azri Ifwat MA, Harun AF, Md Nazir N, Ahmad Radzi MA, Hashim R, et al. Fabrication and characterization of three-dimensional poly (lactic acid-co-glycolic acid), atelocollagen, and fibrin bioscaffold composite for intervertebral disk tissue engineering application. J Bioact Compat Polym. 2017;32:456–68.
Nicholson IP, Gault EA, Foote CG, Nasir L, Bennett D. Human telomerase reverse transcriptase (hTERT) extends the lifespan of canine chondrocytes in vitro without inducing neoplastic transformation. Vet J. 2007;174:570–6.
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52.
Counter CM, Meyerson M, Eaton EN, Ellisen LW, Caddle SD, Haber DA, et al. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–22.
Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, et al. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–8.
Piera-velazquez S, Jimenez SA, Stokes DG. Increased life span of human osteoarthritic chondrocytes by exogenous expression of telomerase. Arthritis Rheum. 2002;46:683–93.
Sun Y, Firestein GS, Wenger L, Huang CY, Cheung HS. Telomerase-transduced osteoarthritic fibroblast-like synoviocyte cell line. Biochem Biophys Res Commun. 2004;323:1287–92.
Xiaoxue Y, Zhongqiang C, Zhaoqing G, Gengting D, Qingjun M, Shenwu W. Immortalization of human osteoblasts by transferring human telomerase reverse transcriptase gene. Biochem Biophys Res Commun. 2004;315:643–51.
Perrault SD, Hornsby PJ, Betts DH. Global gene expression response to telomerase in bovine adrenocortical cells. Biochem Biophys Res Commun. 2005;335:925–36.
Xiang H, Wang J, Mao YW, Li DW. hTERT can function with rabbit telomerase RNA: regulation of gene expression and attenuation of apoptosis. Biochem Biophys Res Commun. 2000;278:503–10.
Kim JH, Do HJ, Yang HM, Oh JH, Choi SJ, Kim DK, et al. Overexpression of SOX9 in mouse embryonic stem cells directs the immediate chondrogenic commitment. Exp Mol Med. 2005;37:261–8.
Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine (Phila Pa 1976). 2003;28:755–63.
Madry H, Trippel SB. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000;7:286–91.
Nabel EG, Yang ZY, Plautz G, Forough R, Zhan X, Haudenschild CC, et al. Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature. 1993;362:844–6.
Nabel EG, Yang Z, Liptay S, San H, Gordon D, Haudenschild CC, et al. Recombinant platelet-derived growth factor B gene expression in porcine arteries induces intimai hyperplasia in vivo. J Clin Invest. 1993;91:1822–9.
Nabel EG, Plautz GE, Nabel GJ. Recombinant growth factor gene expression in vascular cells in vivo. Ann N Y Acad Sci. 1994;714:247–52.
Kim CY, Hwang IK, Kang C, Chung EB, Jung CR, Oh H, et al. Improved transfection efficiency and metabolic activity in human embryonic stem cell using non-enzymatic method. Int J Stem Cells. 2018;11:149–56.
Minegishi Y, Hosokawa K, Tsumaki N. Time-lapse observation of the dedifferentiation process in mouse chondrocytes using chondrocyte-specific reporters. Osteoarthritis Cartilage. 2013;21:1968–75.
Shakibaei M. Integrin expression on epiphyseal mouse chondrocytes in monolayer culture. Histol Histopathol. 1995;10:339–49.
Villar-Suárez V, Calles-Venal I, Bravo IG, Fernández-Alvarez JG, Fernández-Caso M, Villar-Lacilla JM. Differential behavior between isolated and aggregated rabbit auricular chondrocytes on plastic surfaces. J Biomed Biotechnol. 2004;2004:86–92.
Liu Y, Beyer A, Aebersold R. Review on the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165:535–50.
Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2013;13:227–32.
Stokes DG, Liu G, Dharmavaram R, Hawkins D, Piera-Velazquez S, Jimenez SA. Regulation of type-II collagen gene expression during human chondrocyte de-differentiation and recovery of chondrocyte-specific phenotype in culture involves Sry-type high-mobility-group box (SOX) transcription factors. Biochem J. 2001;360:461–70.
Ashraf S, Ahn J, Cha BH, Kim JS, Han I, Park H, et al. RHEB: a potential regulator of chondrocyte phenotype for cartilage tissue regeneration. J Tissue Eng Regen Med. 2017;11:2503–15.
Sasano Y, Furusawa M, Ohtani H, Mizoguchi I, Takahashi I, Kagayama M. Chondrocytes synthesize type I collagen and accumulate the protein in the matrix during development of rat tibial articular cartilage. Anat Embryol (Berl). 1996;194:247–52.
Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27:5990–6003.
Li Y, Meng H, Liu Y, Lee BP. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. ScientificWorldJournal. 2015;2015:685690.
Acknowledgements
The authors thanked the Kulliyyah of Allied Health Sciences (KAHS), IIUM, Kuantan Campus and Ministry of Science, Technology and Innovation (MOSTI) for providing Science Fund (SF14-012-0062). The authors also thanked Tissue Engineering and Regenerative Medicine Research Team, KAHS, IIUM, Kuantan Campus, Assoc. Prof. Dr. Tong Chuan He from University of Chicago, USA for pCDNA3-SOX9 and Dr. Bob Weinberg from Massachusetts Institute of Technology, USA for pBABE-neo-hTERT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Ethical statement
Animal ethical approval was granted by the Institutional Animal Care and Use Committee of International Islamic University Malaysia (IACUC-IIUM) (Reference No. IIUM/IACUC/Approval 2015/[5]/[24]).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Md Nazir, N., Zulkifly, A.H., Khalid, K.A. et al. Matrix Production in Chondrocytes Transfected with Sex Determining Region Y-Box 9 and Telomerase Reverse Transcriptase Genes: An In Vitro Evaluation from Monolayer Culture to Three-Dimensional Culture. Tissue Eng Regen Med 16, 285–299 (2019). https://doi.org/10.1007/s13770-019-00191-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00191-1