Abstract
We experienced a case of a 36-year-old female with rapidly progressive glomerulonephritis (RPGN) due to anti-neutrophil cytoplasmic antibody (ANCA)-associated nephritis and systemic lupus erythematosus (SLE) nephritis. Chiral amino acid metabolomics revealed a prominent profile of d-serine in this patient. At the fulminant period of RPGN, the level of plasma d-serine, a potential biomarker in CKD that reflects actual glomerular filtration ratio (GFR), was extremely high. On the other hand, urinary fractional excretion (FE) of d-serine, which was usually much higher than that of l-isoform, was 0% in this patient. These abnormal d-serine profiles normalized in response to the intensive treatment. Normalizations of blood d-serine levels were in parallel with those of blood creatinine levels and potentially reflect the recovery of GFR. FE of d-serine increased transiently before the normalization of d-serine profile, suggesting that kidney promotes urinary excretion of d-serine for the normalization of plasma d-serine level. These unexplored clinical features of d-serine well reflected the clinical course of this patient. Blood d-serine level can also serve as a biomarker in acute kidney injury (AKI) or RPGN, and, in combination with FE of d-serine, may render the clinical practitioners to judge the efficacy of intensive treatments.
Similar content being viewed by others
Introduction
d-Amino acids, long-term undetected enantiomers of l-amino acids [1,2,3], are now emerging as potential biomarkers for several diseases including kidney diseases [4, 5]. In spite of their trace amount, d-serine does exist in human body [4], and plasma d-serine is now standing out with its usefulness in the estimation of kidney function, glomerular filtration ratio (GFR) [5], and in the prediction of the prognoses of the patients with chronic kidney disease (CKD) [4]. Additionally, urinary fractional excretion (FE) of d-serine turned out to have an association with the presence of kidney diseases [5]. These features of d-serine would potentiate the comprehensive management of CKD.
In light of clinical application, the question if the intra-body dynamics of d-serine reflect the disease course of kidney diseases arises. For example, do the profiles of d-serine reflect worsening or recovery phase of kidney injury? Based on the fact that the profiles of d-serine well correlate with GFR and are associated with the presence of kidney diseases, the profiles of d-serine may sensitively respond to the treatment of kidney diseases. If this is the case, d-serine can be utilized as biomarkers that can also reflect the effects of therapy.
We experienced a case of systemic lupus erythematosus (SLE), who had undergone a severe course of rapid progressive glomerular nephritis that responded well to the intensive care. We report the informative profile of d-serine of this case.
Concise description of this case
Full clinical course of this case is described in the supplemental file. In brief, this case is a 36-year-old woman presented with RPGN. Laboratory test at kidney biopsy showed acute worsening of serum creatinine to 1032 μmol/L, high level of urinary protein (4 g/gCre), strong anemia (blood hemoglobin, 4.6 g/dL), normal levels of complements (C3, 88 mg/dL; C4, 21 mg/dL), positive anti-DNA antibody (13.0 IU/mL), and positive P-ANCA (182.0 U/mL). Kidney biopsy identified crescentic glomerulonephritis, potentially associated with ANCA, and SLE nephritis class V.
A series of plasma exchange was initiated followed by prednisolone pulse, oral prednisolone, intravenous cyclophosphamide, and mycophenolate mofetil. In response to these therapies, serum creatinine level improved to 63.65 μmol/L, while urinary protein level persisted. Followed-up kidney biopsy showed regression of cellular crescents in glomeruli, while 30% of glomeruli were globally sclerosed and capillary thickenings persisted.
Dynamics of d-amino acids in this patient during the recovery phase
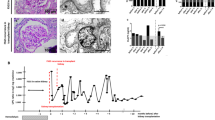
We examined the levels of d-amino acids throughout the recovery phase of RPGN due to SLE nephritis in this patient. Blood levels of d-amino acids at acute phase (just after admission to the hospital) were extraordinary; blood level of d-serine was extremely high and comprised 19% of whole blood serine (ranges of non-CKD were 1.22–1.85%, Fig. 1 and Supplementary Tables S1 and S2). d-Alanine and d-proline, which are often detected in normal population, were also high in this patient; these d-amino acids comprised 2.2% and 3.1% of each amino acid, respectively.
Plasma d-amino acids per total amino acids (D ratio [%]) of this patient on admission. Reference data (non-CKD and CKD) are from [5]. CKD chronic kidney disease
Among d-amino acids, d-serine was demonstrated to reflect GFR [5]. In this patient, blood levels of d-serine decreased in response to the treatment and these decreases were in parallel with those of blood creatinine levels. Finally, blood levels of d-serine further decreased to the normal ranges.
Since FE of d-amino acids was reported to reflect disease profile in CKD [5], we also examined FE of d-amino acids during the clinical course of this patient (Fig. 2a, b). FE of d-serine was calculated as follows: urinary d-serine times blood creatinine divided by urinary creatinine and blood d-serine. FE is the ratio of a substrate filtered by the kidney glomerular that is excreted in the urine. At the initial course of this case, FE of d-serine was 0% due to no excretion of d-serine into the urine. FE of d-serine usually is reported to take wide ranges similarly in both normal and CKD population, whereas 0% of FE of d-serine was completely out of those ranges. FE of d-serine remained 0% until the end of plasma exchange sessions. During the recovery phase, FE of d-serine increased transiently even though the blood levels of both creatinine and d-serine were still high, reflecting the increased excretion of d-serine at this stage. After the series of treatment described here, blood level and FE of d-serine finally normalized to the profile compatible with normal population.
Dynamics of d-serine during the recovery phase of this patient. a Clinical course of this patient. PSL prednisolone, IVCY intravenous cyclophosphamide, MMF mycophenolate mofetil. b Dynamics of d-serine were plotted on a scatter plot with reference data [5]. The eclipse represents 95% confidence interval of non-CKD population. Each numbered dot of this patient reflected the following clinical course: 1, day 1 (on admission); 2, day 9 (before the first plasma exchange); 3, day 13 (before the second plasma exchange); 4, day 30 (after 8 sessions of plasma exchange); 5, day 35 (after IVCY); 6, day 49 (after the initial treatment just before the discharge)
Discussion
We experienced a case of RPGN with dynamic changes in both blood level and FE of d-serine during the recovery phase. At the fulminant phase of RPGN, the blood levels of d-serine were extremely high. On the other hand, FE of d-serine, which was usually much higher than that of l-isoform, was 0% in this patient. These abnormal d-serine profiles normalized in response to the intensive treatment. Normalizations of the blood levels of d-serine were in parallel with those of creatinine. During the recovery phase, FE of d-serine increased transiently and exceeded the normal ranges, followed by a drop in the normal ranges. This dynamic d-serine profile well reflected the clinical course of this patient.
Plasma d-serine may serve as a sensitive marker for AKI and RPGN. Our previous study revealed that d-serine reflects kidney function, GFR, and the presence of CKD [5]. d-Amino acids were known to be handled by kidney; after glomerular filtration, kidney reabsorbs amino acids at proximal tubules with chiral selectivity. In CKD patients with decreased GFR, blood levels of d-serine increased due to less glomerular filtration of d-serine. In line with this study, plasma level of d-serine also reflected recovery phase of RPGN. The abnormally high blood level of d-serine resolved with the treatment of SLE, and the longitudinal course of plasma level of d-serine was in parallel with serum level of creatinine, reflecting the recovery of GFR. Plasma d-serine may be applicable in examining treatment effects in AKI. Additionally, plasma d-serine may also be useful in detecting AKI and RPGN. The presence of a time lag between GFR decrease and blood creatinine increase in AKI is widely known, and whether blood d-serine responds to AKI promptly or not needs to be examined. Once nephrologists all over the world noticed the value of d-serine and started using it, we believe the cost will be reduced very rapidly, and d-serine will be available for daily clinics soon.
On the other hand, the reabsorption of d-serine is sensitive to the presence of CKD [5]. FE of d-serine increases in some patients with normal ranges of blood d-serine level, suggesting that the increment of FE of d-serine is a compensatory mechanism to keep blood d-serine levels low. Another patient showed decreased FE of d-serine, possibly due to decreased GFR, which in turn increased the blood d-serine levels. Therefore, FE of d-serine proceeded the increase of plasma d-serine and turned out as a useful marker for detecting kidney diseases before the worsening of GFR.
This concept was exemplified in this patient; the kidney promotes urinary excretion of d-serine for the normalization of plasma d-serine level. Increase in FE of d-serine during the recovery phase may represent the efficacy of the treatment, a feature essential for the drug discovery in kidney diseases.
In summary, we experienced a case of RPGN due to SLE nephritis accompanied with dynamic profiles of d-serine during its recovery phase. Blood levels of both creatinine and d-serine normalized in response to the treatment, and transient increase in urinary FE of d-serine played a role in normalization. Blood d-serine can serve as a biomarker for AKI and RPGN by reflecting GFR. Those lines of thus-far unexplored course of d-serine suggest the robust utility as a biomarker to monitor disease activity of kidney diseases and the treatment effects.
References
Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, et al. The presence of free d-serine in rat brain. FEBS Lett. 1992;296:33–6.
Krebs HA. Metabolism of amino-acids: deamination of amino-acids. Biochem J. 1935;29:1620–44.
Mothet JP, Parent AT, Wolosker H, Brady RO Jr, Linden DJ, Ferris CD, et al. d-serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA. 2000;97:4926–31.
Kimura T, Hamase K, Miyoshi Y, Yamamoto R, Yasuda K, Mita M, et al. Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci Rep. 2016;6:26137.
Hesaka A, Sakai S, Hamase K, Ikeda T, Matsui R, Mita M, et al. d-Serine reflects kidney function and diseases. Sci Rep. 2019;9:5104.
Acknowledgements
This work was supported by grants from Takeda Science Foundation, from a Grant-in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Society for the Promotion of Science (JSPS) (MEXT/JSPS KAKENHI Grant numbers 17H04188 to TK and YI), and in part by funds and technical supports from Shiseido Co., Ltd. for metabolite measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A part of this study was funded by Shiseido Co., Ltd.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number 16357) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hesaka, A., Yasuda, K., Sakai, S. et al. Dynamics of d-serine reflected the recovery course of a patient with rapidly progressive glomerulonephritis. CEN Case Rep 8, 297–300 (2019). https://doi.org/10.1007/s13730-019-00411-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-019-00411-6