Abstract
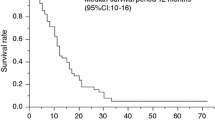
The survival rate and quality of life of patients with recurrent cervical cancer and pleural effusion had been extremely poor until bevacizumab was approved. We report two cases of recurrent cervical cancer with remarkably decreased pleural effusion and a long survival rate after combination chemotherapy with bevacizumab. Case 1: A patient was diagnosed with stage IIB cervical adenocarcinoma and treated with concurrent chemoradiotherapy (CCRT), total hysterectomy, and paclitaxel/carboplatin (TC) therapy as the primary treatment. After the first recurrence had been treated with irinotecan–cisplatin therapy and radiotherapy, symptomatic pleural effusion emerged. Paclitaxel-cisplatin-bevacizumab (Pac-Cis-Bev) was administered during 13 cycles of chemotherapy to promptly relieve pleural effusion, respiratory distress, and back pain. She survived for more than a year and a half after starting Pac-Cis-Bev therapy. Case 2: A patient was diagnosed with stage IIIB cervical squamous cell carcinoma and pulmonary recurrence after CCRT. After 21 cycles of TC or Pac-Cis-Bev therapy, pleural effusion emerged. Topotecan–paclitaxel–bevacizumab (Topo-Pac-Bev) was administered for 12 cycles. Respiratory distress was relieved in 2 weeks and pleural effusion almost completely resolved after 2 months. We changed the treatment to ifosfamide and nedaplatin as pleural effusion exacerbated. However, this treatment was not effective; hence the patient was rechallenged with Topo-Pac-Bev therapy. Six cycles of Topo-Pac-Bev rechallenge therapy effectively suppressed pleural effusion. She survived for 2 years after pleural effusion appeared. Chemotherapy with bevacizumab is useful for both symptom relief and improvement in prognosis in patients with recurrent cervical cancer, despite being in the late phase.







Similar content being viewed by others
References
Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM et al (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370:734–743
Neragi-Miandoab S (2006) Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer 54:1–9
Heffner JE (2008) Diagnosis and management of malignant pleural effusions. Respirology 13:5–20
Kim YJ, Munsell MF, Park JC, Meyer LA, Sun CC, Brown AJ et al (2015) Retrospective review of symptoms and palliative care interventions in women with advanced cervical cancer. Gynecol Oncol 139:553–558
Numnum TM, Rocconi RP, Whitworth J, Barnes MN (2006) The use of bevacizumab to palliate symptomatic ascites in patients with refractory ovarian carcinoma. Gynecol Oncol 102:425–428
Usui K, Sugawara S, Nishitsuji M, Fujita Y, Inoue A, Mouri A et al (2016) A phase II study of bevacizumab with carboplatin-pemetrexed in non-squamous non-small cell lung carcinoma patients with malignant pleural effusions: North East Japan Study Group Trial NEJ013A. Lung Cancer 99:131–136
Fukumura D, Jain RK (2007) Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 74:72–84
Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P et al (1999) Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 85:178–187
Yanagawa H, Takeuchi E, Suzuki Y, Ohmoto Y, Bando H, Sone S (1999) Vascular endothelial growth factor in malignant pleural effusion associated with lung cancer. Cancer Immunol Immunother 48:396–400
Duda DG, Willett CG, Ancukiewicz M, di Tomaso E, Shah M, Czito BG et al (2010) Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist 15:577–583
dos Santos LV, Cruz MR, Lopes GD, Lima J (2015) VEGF-A levels in bevacizumab-treated breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat 151:481–489
Tamiya M, Tamiya A, Yasue T, Nakao K, Omachi N, Shiroyama T et al (2016) Vascular endothelial growth factor in plasma and pleural effusion is a biomarker for outcome after bevacizumab plus carboplatin-paclitaxel treatment for non-small cell lung cancer with malignant pleural effusion. Anticancer Res 36:2939–2944
Bodily JM, Mehta KPM, Laimins LA (2011) Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Can Res 71:1187–1195
Clere N, Bermont L, Fauconnet S, Lascombe I, Saunier M, Vettoretti L et al (2007) The human papillomavirus type 18 E6 oncoprotein induces vascular endothelial growth factor 121 (VEGF(121)) transcription from the promoter through a p53-independent mechanism. Exp Cell Res 313:3239–3250
Cheng WF, Chen CA, Lee CN, Wei LH, Hsieh FJ, Hsieh CY (2000) Vascular endothelial growth factor and prognosis of cervical carcinoma. Obstet Gynecol 96:721–726
Sawada M, Oishi T, Komatsu H, Sato S, Chikumi J, Nonaka M et al (2019) Serum vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 as prognostic biomarkers for uterine cervical cancer. Int J Clin Oncol 24:1612–1619
Moore DH, Tian CQ, Monk BJ, Long HJ, Omura GA, Bloss JD (2010) Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol 116:44–49
Shoji T, Komiyama S, Kigawa J, Tanabe H, Kato K, Itamochi H et al (2018) An open-label, randomized, phase II trial evaluating the efficacy and safety of standard of care with or without bevacizumab in platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer patients previously treated with bevacizumab for front-line or platinum-sensitive ovarian cancer: rationale, design, and methods of the Japanese Gynecologic Oncology Group study JGOG3023. BMC Cancer. https://doi.org/10.1186/s12885-018-4505-4
Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM et al (2017) Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 390:1654–1663
Seebacher V, Sturdza A, Bergmeister B, Polterauer S, Grimm C, Reinthaller A et al (2019) Factors associated with post-relapse survival in patients with recurrent cervical cancer: the value of the inflammation-based Glasgow Prognostic Score. Arch Gynecol Obstet 299:1055–1062
Acknowledgements
We would like to thank Takashi Hibiya M.D., Ph.D., Akio Miyake M.D., Yukiko Nishio C.T., Chinami Hoshino C.T., and Hiromi Sagawa C.T. for cytological examination. We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
NK wrote the manuscript. TM contributed to writing the manuscript. TM and EM supervised the study and revised the manuscript. NK and TM served as attending physicians of the present patients. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest as to this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from the patients for the publication of this case report and its accompanying images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kamiya, N., Matsunaga, T. & Miyagi, E. Two cases showing the effects of bevacizumab on recurrent cervical cancer with pleural effusion. Int Canc Conf J 11, 165–171 (2022). https://doi.org/10.1007/s13691-022-00538-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-022-00538-x