Abstract
Here, we report a 57-year-old female patient with HER2-positive recurrent gastric cancer who experienced drug-induced thrombocytopenia associated with trastuzumab, a humanized anti-HER2 monoclonal antibody. Shortly after the initiation of S-1, oxaliplatin, and trastuzumab chemotherapy, the patient experienced severe thrombocytopenia and did not respond to platelet transfusions. Based on the findings of increased numbers of polynuclear megakaryocytes in the bone marrow and an elevated level of platelet-associated IgG (PA-IgG), the patient was diagnosed with drug-induced thrombocytopenia (DITP). The platelet count recovered rapidly with oral prednisolone (1 mg/kg). Since we initially suspected oxaliplatin as the causal agent, S-1 was restarted as a monotherapy, followed by trastuzumab after a 3-week interval, without oxaliplatin. On the second day after the addition of trastuzumab, severe thrombocytopenia occurred again, which suggests that trastuzumab was responsible for the DITP. The patient no longer experienced severe thrombocytopenia during the subsequent S-1 and oxaliplatin chemotherapy, which supports this hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The humanized anti-human epidermal growth factor 2 (HER2) monoclonal antibody trastuzumab is a key drug in first-line chemotherapy regimens for HER2-positive advanced gastric or gastro-esophageal junction cancers. Trastuzumab occasionally causes hypersensitivity reactions, also known as infusion reactions, and latent or overt cardiac dysfunction after long-term treatment, whereas this drug rarely causes hematological toxicity or myelosuppression, which are common among cytotoxic anticancer drugs. Here, we report a case of acute, severe thrombocytopenia that was likely associated with trastuzumab.
Case presentation
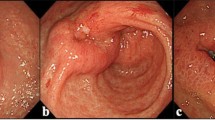
A 57-year-old female patient was admitted to our hospital for swollen para-aortic lymph nodes detected by abdominal ultrasonography during a routine medical checkup. Thirteen years earlier, the patient had undergone total gastrectomy for T1bN1M0 Stage IB (AJCC 7th edition) gastric cancer. The patient did not receive adjuvant chemotherapy. She was also treated with entecavir for chronic hepatitis B and denosumab for osteoporosis and had received mecobalamin as an oral vitamin B12 supplement after total gastrectomy. H. pylori infection was not assessed. She also had no history of allergic reaction to medications or substances. A pathologic examination of CT-guided needle biopsy specimens from the para-aortic lymph nodes revealed HER2-positive moderately differentiated adenocarcinoma that was consistent with the metastatic lymph nodes from gastric cancer 13 years earlier (Fig. 1a). The patient was then diagnosed with HER2-positive recurrent gastric cancer.
The patient began treatment with first-line chemotherapy consisting of oral S-1 (a combination of tegafur, gimeracil, and oteracil potassium) at a dose of 50 mg twice daily on a 2-week on and 1-week off schedule, intravenous oxaliplatin (100 mg/m2), and trastuzumab (8 mg/kg as a loading dose followed by 6 mg/kg; CT-P6, Nippon Kayaku, Tokyo, Japan) given on day 1 of a 3-week cycle. Platelet count was 293,000/µL, and the other blood chemistry and hematologic findings were within the normal range at the time of chemotherapy initiation. Oxaliplatin and trastuzumab infusions were successfully completed; however, a grade 1 infusion reaction occurred during trastuzumab infusion. The patient was discharged the following day (day 2). At a follow-up outpatient visit on day 8, purpura and petechiae that appeared shortly after discharge were observed throughout the patient's body. She was also aware of macrohematuria that was persistent since day 3. Hematologic tests revealed grade 4 thrombocytopenia, a platelet count of 1000/µL, and moderate anemia with a hemoglobin level of 8.1 g/dL, while other blood chemistry and hematologic findings, such as white blood cell counts and neutrophil to lymphocyte ratio, were within normal range. According to the patient and her family, S-1 had been suspended since day 2.
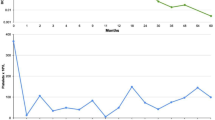
The patient was hospitalized immediately and received repeated platelet transfusions, but her platelet count did not recover (Fig. 2a). Based on the findings of increased numbers of polynuclear megakaryocytes in the bone marrow and an elevated level of platelet-associated IgG (PA-IgG, 96 ng/107 cells; normal range is less than 46 ng/107 cells), the patient was diagnosed with drug-induced thrombocytopenia (DITP). After starting oral prednisolone (1 mg/kg) on day 10, the platelet count recovered rapidly: 74,000/µL on day 12 and 179,000/µL on day 14. Since we initially suspected oxaliplatin as a causal drug, S-1 was restarted as a monotherapy, followed by trastuzumab at a dose of 8 mg/kg after a 3-week interval, without oxaliplatin. No infusion reaction was observed during or immediately after trastuzumab infusion, and the administration was completed safely. However, on the following day, purpura and petechiae reappeared throughout the patient's body, and hematologic tests revealed severe thrombocytopenia with a platelet count of 1000/µL, which suggests that trastuzumab was responsible for the DITP (Fig. 2b). Since a significant tumor response to treatment was recognized on follow-up CT imaging (Fig. 1b), the patient continued S-1 and oxaliplatin treatment and no longer experienced severe thrombocytopenia.
Discussion
DITP is a rare adverse event associated with antibody-mediated platelet destruction that is triggered by exposure to a causal drug. [1,2,3] Among several proposed mechanisms of DITP, it is highly probable that autoantibodies specific for glycoproteins (IIb/IIIa) on the platelet surface caused the destruction of platelets in the circulation of this patient. Besides the elevated levels of PA-IgG, [4] trastuzumab re-challenge immediately reproduced thrombocytopenia, which is consistent with the immune mechanism of DITP.
More than 300 drugs and agents, including oxaliplatin [5, 6], have been reported to cause DITP [1,2,3]. Thirteen cases of trastuzumab-related DITP have been reported thus far, including the present case (Table 1) [7,8,9,10,11,12,13,14,15,16,17,18,19]. The present case differs from other reported cases in that our patient had gastric cancer, and to our knowledge, this is the only case treated with the trastuzumab biosimilar CT-P6. According to the manufacturer (Nippon Kayaku, Tokyo, Japan), no incidents of DITP associated with this trastuzumab biosimilar have been reported other than our case. It is unknown whether biosimilar exhibit cross-reactivity to reference trastuzumab.
In conclusion, we report the first case of DITP associated with a trastuzumab biosimilar in a patient with gastric cancer, which provides useful insight into the practical management of DITP in molecular-targeted cancer treatment.
References
Aster RH, Bougie DW (2007) Drug-induced immune thrombocytopenia. N Engl J Med 357(6):580–587
Bakchoul T, Marini I (2018) Drug-associated thrombocytopenia. Hematology Am Soc Hematol Educ Program 2018(1):576–583
Warkentin TE (2007) Drug-induced immune-mediated thrombocytopenia–from purpura to thrombosis. N Engl J Med 356(9):891–893
Kelton JG, Meltzer D, Moore J et al (1981) Drug-induced thrombocytopenia is associated with increased binding of IgG to platelets both in vivo and in vitro. Blood 58(3):524–529
Jardim DL, Rodrigues CA, Novis YAS, Rocha VG, Hoff PM (2012) Oxaliplatin-related thrombocytopenia. Ann Oncol 23(8):1937–1942
Pan E, Hsieh E, Piatek C (2018) Case Report: oxaliplatin-induced immune-mediated thrombocytopenia. Case Rep Oncol 11(3):880–882
Cathomas R, Goldhirsch A, von Moos R (2007) Drug-induced immune thrombocytopenia. N Engl J Med 357(18):1870–1871
Parikh O, Neave F, Palmieri C (2008) Severe thrombocytopenia induced by a single infusion of trastuzumab. Clin Breast Cancer 8(3):285–286
Cathomas R, von Moos R (2009) Severe drug-induced thrombocytopenia after treatment with trastuzumab but not with lapatinib. Ann Oncol 20(9):1606–1607
Jara Sánchez C, Olier Gárate C, García-Donas Jiménez J, Peñalver PJ (2009) Drug-induced thrombocytopenia induced by trastuzumab: a special challenge in a curable disease. Ann Oncol 20(9):1607–1608
Drudi F, Gianni L, Fantini M, Ravaioli A (2010) Trastuzumab-related thrombocytopenia: always a self-limiting complication? Ann Oncol 21(3):668–669
Mantzourani M, Gogas H, Katsandris A, Meletis J (2011) Severe thrombocytopenia related to trastuzumab infusion. Med Sci Monit 17(7):CS85-87
Aguirre E, Taberner T, Luaña A, Morales S, Llombart A (2013) Severe thrombocytopenia related to long-term trastuzumab exposure. Tumori 99(1):e1-2
Pino MS, Angiolini C, Fioretto L (2013) Severe thrombocytopenia after trastuzumab retreatment: a case report. BMC Res Notes 6:400
Zeng RC, Dai XX, Xie FY, Chen ED, Qu JM, Hu XQ (2014) Severe thrombocytopenia induced by second exposure to trastuzumab can be alleviated by prolonging the interval between treatments. Clin Breast Cancer 14(2):e69–e72
Miarons M, Velasco M, Campins L, Fernández S, Gurrera T, Lopez-Viaplana L (2016) Gradual thrombocytopenia induced by long-term trastuzumab exposure. J Clin Pharm Ther 41(5):563–565
Luo Q, Guo Z, Ye C (2019) Thrombocytopenia Induced by Herceptin. Am Surg 85(4):e222–e224
Zhou Q, Dong J, Jiang X, Pan Y, Han X (2020) Trastuzumab-induced thrombocytopenia after eight cycles of trastuzumab treatment. Open Med (Wars) 15(1):659–662
Wang X, Zhu X, Zou J, Zhang X, Kong X, Nie J (2021) Severe thrombocytopenia induced by trastuzumab rechallenge: a case report and literature review. J Clin Pharm Ther 46:1173–1177
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yuichi Ando received a speaker honorarium from Chugai Pharmaceutical Co., Ltd. and research funding s from Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co.,Ltd., Ono Pharmaceutical Co., Ltd., Novartis Pharma K.K., Bayer Holding Ltd., and Geo Holding Corporation. Other authors have no conflict of interest.
Informed consent
Written informed consent was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Takano, Y., Furune, S., Miyai, Y. et al. Drug-induced thrombocytopenia associated with trastuzumab in a patient with HER2-positive recurrent gastric cancer. Int Canc Conf J 11, 67–70 (2022). https://doi.org/10.1007/s13691-021-00520-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-021-00520-z