Abstract
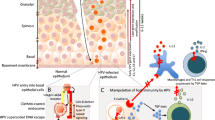
Human papillomavirus (HPV) infection in the genital tract is common in young sexually active individuals, the majority of whom clear the infection without overt clinical disease. However, most of those who develop benign lesions eventually mount an effective cell-mediated immune response and the lesions regress. Regression of anogenital warts is accompanied histologically by a CD4+ T cell-dominated Th1 response, and animal models support this and provide evidence that the response is modulated by antigen-specific CD4+ T cell-dependent mechanisms. Failure to develop effective CMI to clear or control infection results in persistent infection and, in the case of the oncogenic HPVs, an increased probability of progression to high-grade intraepithelial neoplasia and invasive carcinoma. Effective evasion of innate immune recognition and activation of adaptive immune responses seem to be the hallmark of HPV infections. The viral infectious cycle is exclusively intraepithelial, there is no viraemia, no virus-induced cytolysis or cell death and viral replication, and release is not associated with inflammation. HPV globally downregulates the innate immune signalling pathways in the infected keratinocyte, pro-inflammatory cytokines particularly the type I interferons are not released, and the signals for Langerhans cell (LC) activation and migration together with recruitment of stromal dendritic cells and macrophages are either not present or inadequate. This immune ignorance results in chronic infections that persist over weeks and months. Progression to high-grade intraepithelial neoplasia with the concomitant upregulation of the E6 and E7 oncoproteins is associated with further deregulation of immunologically relevant molecules, particularly chemotactic chemokines and their receptors, on keratinocytes and endothelial cells of the underlying microvasculature limiting or preventing the ingress of cytotoxic effectors into the lesions.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30 Suppl 5:F12–23.
de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–15.
Ball SL, Winder DM, Vaughan K, Hanna N, Levy J, Sterling JC, et al. Analyses of human papillomavirus genotypes and viral loads in anogenital warts. J Med Virol. 2011;83(8):1345–50.
Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30 Suppl 5:F55–70.
Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–14.
Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14(5):289–301.
Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25(2):215–22.
Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679–91.
Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–26.
Nasu K, Narahara H. Pattern recognition via the toll-like receptor system in the human female genital tract. Mediat Inflamm. 2010;2010:976024.
Loo YM, Gale Jr M. Immune signaling by RIG-I-like receptors. Immunity. 2011;34(5):680–92.
Taylor KE, Mossman KL. Recent advances in understanding viral evasion of type I interferon. Immunology. 2013;138(3):190–7.
Black AP, Ardern-Jones MR, Kasprowicz V, Bowness P, Jones L, Bailey AS, et al. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur J Immunol. 2007;37(6):1485–93.
Karim R, Meyers C, Backendorf C, Ludigs K, Offringa R, van Ommen GJ, et al. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One. 2011;6(3):e17848. The first description using virus-infected keratinocytes to show a global downregulation of keratinocyte-generated antiviral and pro-inflammatory cytokines.
Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2013;13(1):46–57.
Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J Virol. 2001;75(9):4283–96.
Karstensen B, Poppelreuther S, Bonin M, Walter M, Iftner T, Stubenrauch F. Gene expression profiles reveal an upregulation of E2F and downregulation of interferon targets by HPV18 but no changes between keratinocytes with integrated or episomal viral genomes. Virology. 2006;353(1):200–9.
Chang YE, Laimins LA. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J Virol. 2000;74(9):4174–82.
Tummers B, Burg SH. High-risk human papillomavirus targets crossroads in immune signaling. Virus. 2015;7(5):2485–506.
Coiera E, Moerman-Herzog A, Nakagawa M. Early defensive mechanisms against human papillomavirus infection. J Med Internet Res. 2015;22:850–7.
Reiser J, Hurst J, Voges M, Krauss P, Munch P, Iftner T, et al. High-risk human papillomaviruses repress constitutive kappa interferon transcription via E6 to prevent pathogen recognition receptor and antiviral-gene expression. J Virol. 2011;85(21):11372–80.
Sunthamala N, Thierry F, Teissier S, Pientong C, Kongyingyoes B, Tangsiriwatthana T, et al. E2 proteins of high risk human papillomaviruses down-modulate STING and IFN-kappa transcription in keratinocytes. PLoS One. 2014;9(3):e91473.
Muto V, Stellacci E, Lamberti AG, Perrotti E, Carrabba A, Matera G, et al. Human papillomavirus type 16 E5 protein induces interferon-beta expression through interferon regulatory factor-1 in human keratinocytes. J Virol. 2011;85(10):5070–80.
Karim R, Tummers B, Meyers C, Biryukov JL, Alam S, Backendorf C, et al. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte’s innate immune response. PLoS Pathog. 2013;9(5):e1003384.
Tummers B, Goedemans R, Pelascini LP, Jordanova ES, van Esch EM, Meyers C, et al. The interferon-related developmental regulator 1 is used by human papillomavirus to suppress NFkappaB activation. Nat Commun. 2015;6:6537.
Fausch SC, Da Silva DM, Rudolf MP, Kast WM. Human papillomavirus virus-like particles do not activate Langerhans cells: a possible immune escape mechanism used by human papillomaviruses. J Immunol. 2002;169(6):3242–9.
Da Silva DM, Fausch SC, Verbeek JS, Kast WM. Uptake of human papillomavirus virus-like particles by dendritic cells is mediated by Fcgamma receptors and contributes to acquisition of T cell immunity. J Immunol. 2007;178(12):7587–97.
Devoti J, Hatam L, Lucs A, Afzal A, Abramson A, Steinberg B, et al. Decreased Langerhans cell responses to IL-36gamma: altered innate immunity in patients with recurrent respiratory papillomatosis. Mol Med. 2014;20(1):372–80.
Matthews K, Leong CM, Baxter L, Inglis E, Yun K, Backstrom BT, et al. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J Virol. 2003;77(15):8378–85.
Leong CM, Doorbar J, Nindl I, Yoon HS, Hibma MH. Deregulation of E-cadherin by human papillomavirus is not confined to high-risk, cancer-causing types. Br J Dermatol. 2010;163(6):1253–63.
van Seters M, Beckmann I, Heijmans-Antonissen C, van Beurden M, Ewing PC, Zijlstra FJ, et al. Disturbed patterns of immunocompetent cells in usual-type vulvar intraepithelial neoplasia. Cancer Res. 2008;68(16):6617–22.
Guess JC, McCance DJ. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J Virol. 2005;79(23):14852–62.
Nakayama Y, Asagoe K, Yamauchi A, Yamamoto T, Shirafuji Y, Morizane S, et al. Dendritic cell subsets and immunological milieu in inflammatory human papilloma virus-related skin lesions. J Dermatol Sci. 2011;63(3):173–83.
Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30 Suppl 5:F24–33.
Nicholls PK, Moore PF, Anderson DM, Moore RA, Parry NR, Gough GW, et al. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+ lymphocytes. Virology. 2001;283(1):31–9.
Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102(6):768–74.
de Jong A, van der Burg SH, Kwappenberg KM, van der Hulst JM, Franken KL, Geluk A, et al. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002;62(2):472–9.
Woo YL, van den Hende M, Sterling JC, Coleman N, Crawford RA, Kwappenberg KM, et al. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer. 2010;126(1):133–41.
Jain S, Moore RA, Anderson DM, Gough GW, Stanley MA. Cell-mediated immune responses to COPV early proteins. Virology. 2006;356(1-2):23–34.
McKenzie J, King A, Hare J, Fulford T, Wilson B, Stanley M. Immunocytochemical characterization of large granular lymphocytes in normal cervix and HPV associated disease. J Pathol. 1991;165(1):75–80.
Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185(11):7107–14.
Hubert P, Herman L, Roncarati P, Maillard C, Renoux V, Demoulin S, et al. Altered alpha-defensin 5 expression in cervical squamocolumnar junction: implication in the formation of a viral/tumour-permissive microenvironment. J Pathol. 2014;234(4):464–77.
Wiens ME, Smith JG. Alpha-defensin HD5 inhibits furin cleavage of human papillomavirus 16 L2 to block infection. J Virol. 2015;89(5):2866–74.
Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118(1 Suppl):S12–7.
Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–44.
Remoue F, Jacobs N, Miot V, Boniver J, Delvenne P. High intraepithelial expression of estrogen and progesterone receptors in the transformation zone of the uterine cervix. Am J Obstet Gynecol. 2003;189(6):1660–5.
Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–9.
Fung KY, Mangan NE, Cumming H, Horvat JC, Mayall JR, Stifter SA, et al. Interferon-epsilon protects the female reproductive tract from viral and bacterial infection. Science. 2013;339(6123):1088–92.
Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis. 2014;210(11):1723–33.
Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect. 2015;21(7):674–E1-9.
Dareng EO, Ma B, Famooto AO, Akarolo-Anthony SN, Offiong RA, Olaniyan O, et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect. 2015;11:1–15.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Margaret Stanley declares that she received personal fees for being a consultant to MSD Merck, GSK Biologicals and SPMSD.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Management of HPV and Associated Cervical Lesions
Rights and permissions
About this article
Cite this article
Stanley, M. Immunology of HPV Infection. Curr Obstet Gynecol Rep 4, 195–200 (2015). https://doi.org/10.1007/s13669-015-0134-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-015-0134-y