Abstract
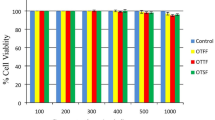
To study the α-amylase and α-glucosidase enzymes inhibitory activity in vitro of ethanol (PLEE) and chloroform (PLCE) extracts of Polyalthia longifolia (Sonner.) Thw. leaves and in vivo antidiabetic activity against streptozotocin-induced type 1 diabetes mellitus in rats. In vitro studies were performed using α-amylase and α-glucosidase enzymes followed by enzyme kinetics using Lineweaver-Burk plot analysis. Acute toxicity studies were performed as per OECD guidelines 423. Streptozotocin (60 mg/kg b.w., i.p.) was used to induce type 1 diabetes mellitus in rats. Changes in body weight changes, blood glucose levels, serum marker enzymes, serum lipid profile, enzymatic and non-enzymatic antioxidants in liver homogenates were measured and histopathology of pancreatic tissues were done. The IC50 of PLEE for α-amylase was found to be 154.3 ± 2.42 μg/ml whereas PLCE was 180.3 ± 1.35 μg/ml. The IC50 values of the PLEE for α-glucosidase inhibition was found to be 208.7 ± 2.54 μg/ml and PLCE showed at 271.6 ± 0.85 μg/ml. Acute toxicity studies showed that the extracts were safe at 2000 mg/kg b.w. Both the extracts dose dependently reversed the abnormal changes observed in untreated diabetic rats. The effect produced by the ethanol extract was slightly higher than the chloroform extract. The results of this present study suggest that the ethanol and chloroform extracts of leaves of Polyalthia longifolia showed α-amylase and α-glucosidase enzymes inhibitory activity. The effect was further highlighted by the protection of the extracts against streptozotocin-induced type 1 diabetes mellitus in rats.



Similar content being viewed by others
References
American Diabetes Association (2008) Diagnosis and classification of diabetes mellitus. Diabetes Care 31:S55–S60
Bachhawat AJ, Shihabudeen MS, Thirumurugan K (2011) Screening of fifteen Indian ayurvedic plants for alpha-glucosidase inhibitory activity and enzyme kinetics. Int J Pharm Pharmaceut Sci 3:267–274
Baker H, Frank O, DeAngelis B, Feingold S (1980) Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Rep Int 1:531–536
Brownlee M, Cerami A, Vlassara H (1988) Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 318:1315–1321
Chen C, Chang F, Shih Y, Hseih T, Chia Y, Tseng H et al (2000) Cytotoxic Constituents of Polyalthia longifolia var. pendula. J Nat Prod 63:1475–1478
Chethan S, Sreerama YN, Malleshi NG (2008) Mode of inhibition of finger millet malt amylases by the millet phenolics. Food Chem 111:187–191
Devasagayam TPA, Boloor KK, Ramasarma T (2003) Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophy 40:300–308
Dey L, Attel AS, Yuan C (2002) Alternative therapies for type 2 diabetes. Altern Complement Ther 7:45–58
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 2:70–77
Erejuwa OO, Sulaiman SA, Wahab MS, Sirajudeen KNS, Salleh MS, Gurtu S (2012) Hepatoprotective effect of tualang honey supplementation in streptozotocin-induced diabetic rats. Int J App Res Nat Prod 4(4):37–41
Goldin A, Beckman JA, Schmidt AM, Creager MA (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114(6):597–605
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylases: a biotechnological perspective. Process Biochem 38:1599–1616
Harris EH (2005) Elevated liver function tests in type 2 diabetes. Clin Diabetes 23(3):115–119
Howard BV (1987) Lipoprotein metabolism in diabetes mellitus. J Lipid Res 28:613–628
International Diabetes Federation (2011) IDF Diabetes Atlas, 5th edn. International Diabetes Federation, Brussels
Jain AK, Jain A, Jain S, Sikarwar MS, Dubey SK (2006) Hepatoprotective activity of ethanolic extracts of leaves of Polyalthia longifolia. Plant Arch 6:841–842
Jing M, Rayner CK, Jones KL, Horowitz Z (2009) Insulin secretion in healthy subjects and patients with Type 2 diabetes—role of the gastrointestinal tract. Best Pract Res Clin Endocrinol Metab 23:413–424
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 2:130–132
Kritikar KR, Basu BD (1987) Indian medicinal plants, Vol I and II, 2nd edn. International book distributors, Dehradun
Kumar KA, Umamaheswari M, Sivashanmugam AT, Subhadradevi V, Somanathan SS, Ravi TK (2009) Protective effect of Asystasia gangetica reduced oxidative damage in the small intestine of streptozotocin-induced diabetic rats. Orient Pharm Exp Med 9(4):307–314
Lobarzewski J, Ginalska J (1995) Industrial use of soluble or immobilized plant peroxidases. Plant Perox Newslett 6:3–7
Lowry OH, Rosenbourgh NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Maarel MJEC, Veen B, Uitdehaag JCM, Leemhuis H, Dijkhuizen L (2002) Properties and applications of starch converting enzymes of the α-amylase family. J Biotechnol 94:137–155
Malairajan P, Gopalakrishnan G, Narasimhan S, Veni KJK (2008) Evaluation of antiulcer activity of Polyalthia longifolia (Sonn) Thwaites in experimental animals. Indian J Pharmacol 40:126–128
McAnuff MA, Omoruyi FO, Morrison EY, Asemota HN (2005) Changes in some liver enzymes in streptozotocin-induced diabetic rats fed sapogenin extract from bitter yam (Dioscorea polygonoides) or commercial diosgenin. West Indian Med J 54(2):97–101
Moller N, Nair KS (2008) Diabetes and protein metabolism. Diabetes 57:3–4
Nair R, Chanda S (2006a) Activity of some medicinal plants against certain pathogenic bacterial strains. Indian J Pharmacol 38:142–144
Nair R, Chanda S (2006b) Evaluation of Polyalthia longifolia (Sonn.) Thw. leaf extract for antifungal activity. J Cell Tissue Res 6:581–584
O’Brien RM, Granner DK (1991) Regulation of gene expression by insulin. Biochem J 278:609–619
OECD, Test No. 423: Acute Oral toxicity—Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing; (2002) doi:10.1787/9789264071001-en
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxides. J Lab Clin Med 70:158–159
Powers AC, D’Alessio (2011) Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycaemia. In: Brunton LL, Chabner BA, Knollmann BC (eds) Goodman and Gillman’s: The Pharmacological basis of therapeutics, 12th edn. McGrawHill Medical Publishing Division, New York, pp 1237–1274
Pushparaj PN, Low HK, Manikandan J, Tan BK, Tan CH (2007) Antidiabetic effects of Cichorium intybus in streptozotocin-induceddiabetic rats. J Ethnopharmacol 111:430–434
Racker E (1952) Enzymatic synthesis and breakdown of desoxyribose phosphate. J Biol Chem 196:347–365
Rajkumar L, Srinivasan N, Balasubramanian K, Govindarajulu P (1991) Increased degradation of dermal collagen in diabetic rats. Indian J Exp Biol 29:1081–1083
Rao TN, Kumarappan C, Thilagam E, Mohanalakshmi S, Subash MC (2008) Inhibition of carbohydrate digestive enzymes by Talinum portulacifolium (Forssk) leaf extract. J Complement Integr Med 5:1–10
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Sivashanmugam AT, Chatterjee TK (2011) In vitro evaluation of free radical scavenging activity of medicinal plant Polyalthia longifolia (Sonner.) Thw. leaf extract. J Pharm Res 4:3776–3780
Sivashanmugam AT, Chatterjee TK (2012a) Anticataractogenesis activity of Polyalthia longifolia leaves extracts against glucose-induced cataractogenesis using goat lenses in vitro. European J Exp Biol 2:105–113
Sivashanmugam AT, Chatterjee TK (2012b) Xanthine oxidase inhibitory activity and enzyme kinetics of Polyalthia longifolia (Sonner.) Thw. leaves using in vitro method. Int J Biol Pharm Res 3:61–65
Smith BW, Adams LA (2011) Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol 7(8):456–465
Subramanian R, Asmawi MZ, Sadikun A (2008) In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim Pol 55:391–398
Sun F, Iwaguchi K, Shudo R, Nagaki Y, Tanaka K, Ikeda K et al (1999) Change in tissue concentrations of lipid hydroperoxides, vitamin C and vitamin E in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 96(2):185–190
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in β cells of the rat pancreas. Physiol Res 50:536–546
Wadkar KA, Magdum CS, Patil SS, Naikwade NS (2008) Antidiabetic potential and Indian medicinal plants. J Herb Med Toxicol 2:45–50
Winkler R, Moser M (1992) Alterations of antioxidant tissue defense enzymes and related metabolic parameters in streptozotocin-diabetic rats–effects of iodine treatment. Wien Klin Wochenschr 104(14):409–413
Wu Y, Duh C (1990) Two new natural azafluorene alkaloids and a cytotoxic aporphine alkaloid from Polyalthia longifolia. J Nat Prod 53:1327–1331
Yazdanparast R, Esmaeili MA, Helan JA (2005) Teucrium polium extract effects pancreatic function of streptozotocin diabetic rats: a histopathological examination. Iran Biomed J 9(2):81–85
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivashanmugam, A.T., Chatterjee, T.K. In vitro and in vivo antidiabetic activity of Polyalthia longifolia (Sonner.) Thw. leaves. Orient Pharm Exp Med 13, 289–300 (2013). https://doi.org/10.1007/s13596-013-0118-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-013-0118-2