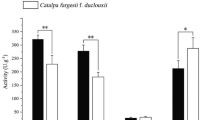Abstract
Key message
Our findings highlight that male-rooted clones (M/M and F/M) of Populus deltoides are more water-deficit tolerant than female-rooted clones (F/F and M/F), as shown by a smaller decrease in total biomass and net photosynthetic rate in the male-rooted clones.
Context
It has been reported that graft clones (female scion and male rootstock) in poplars may be an option to mitigate the effects of water deficit on plants. However, the extent to which grafting mitigates the effect of soil water deficit on soil microorganisms remains poorly understood.
Aims
The research was designed to investigate the effects of soil water deficit on plant growth, soil microbial communities, and soil enzymatic activities of the reciprocal graft clones of P. deltoides.
Methods
Four different graft clones (female shoot scion and female rootstock, F/F; male shoot scion and female rootstock, M/F; male shoot scion and male rootstock scion, M/M; and female shoot scion and male rootstock, F/M) in P. deltoides were used. Two watering regimes, irrigation and soil water deficit (i.e., non-irrigation conditions, natural levels of precipitation) were included in the experiments. The microbial community structure was quantified using phospholipid fatty acid.
Results
Under water deficit, the decreases in total biomass, net photosynthetic rates, and leaf nitrogen content were more evident in M/M and F/M than in F/F and M/F. Bacterial phospholipid fatty acids (PLFAs) and actinomycetal PLFAs remained unaffected in the four graft clones. In contrast, during soil water deficit, fungal PLFAs were higher in M/M and F/M soil coincided with higher extracellular activities of β-1,4-N-acetyl-glucosaminidase and leucine aminopeptidase.
Conclusion
Male-rooted clones (M/M and F/M) are more tolerant to water deficit than female-rooted clones (F/F and M/F). It is possible that the better performance of M/M and F/M, when exposed to water deficit, is associated mainly with higher fine root activity, greater specific root length and root/shoot ratio as well as increased fungal PLFAs.
Similar content being viewed by others
1 Introduction
Water availability is one of the most important factors affecting plant growth, survival, distribution and forest production in many regions of the world (Ciais et al. 2005; Allen et al. 2010; Scheffers et al. 2016). Grafting may be an option to mitigate environmental stresses on plant growth (Schwarz et al. 2010; Machado et al. 2013; Cantero-Navarro et al. 2016; Han et al. 2018). Rootstocks affect plant growth and modulate leaf water potential, stomatal conductance, and photosynthesis under water deficit (Martínez-Ballesta et al. 2010; Rodríguez-Gamir et al. 2010; Han et al. 2018). Numerous studies have examined the effects of grafting on plant growth under abiotic stress conditions (Martinez-Rodriguez et al. 2008; Rouphael et al. 2008; Schwarz et al. 2010; Han et al. 2013; Machado et al. 2013; Han et al. 2018). However, these studies tend to focus on aboveground plant responses and traits. To date, relatively little attention has been given to the belowground parts (Zhou et al. 2010). Further, most previous studies of grafting have focused on horticultural plants (Rouphael et al. 2008; Erismann et al. 2008; Colla et al. 2010; Louws et al. 2010; Martínez-Ballesta et al. 2010; Rodríguez-Gamir et al. 2010). Thus far, only limited studies on the effects of soil water depletion in grafted forestry trees have been reported (Han et al. 2018).
Soil microorganisms play a crucial role in the availability of nutrients for plant growth. Soil water depletion poses direct impacts on soil microbial communities and microbial activities because several microbial taxonomic groups such as bacteria, fungi, actinobacteria, and arbuscular mycorrhizal fungi (AMF) are known to show different sensitivity to water deficit (Hawkes et al. 2011; Mariotte et al. 2015). Additionally, numerous studies have shown changes in individual tree species may influence soil microbial communities and nutrient availability through litter quality, root mass and exudates (Paterson et al. 2009; Phillips et al. 2012; Fender et al. 2013). Root exudates of rootstock are different in composition from that of the scion, and a small change in root exudates may lead to large alterations in the composition of the microbiome (Tian et al. 2009), Thus, grafting may influence soil microbial communities through root exudates (Gao et al. 2015). Although both water and grafting are likely to affect soil microbial communities, information on the influences of water deficit on microbial communities and microbially mediated processes in grafted plants is lacking (Karlowsky et al. 2018); in particular, the extent to which grafting mitigates the effect of water deficit on soil microorganisms remains poorly understood.
Poplars are important trees in China because of their fast growth and light soft wood. Poplars are distributed mainly in semi-arid regions with frequent water deficit ,which is an emerging threat to forest production under climate change. Populus cathayana males are more tolerant to abiotic stress than females (Xu et al. 2008; Zhang et al. 2011; Zhao et al. 2012). Furthermore, Han et al. (2013) reported that the combination of female shoot scion/male rootstock (F/M) in P. cathayana showed a significantly higher total biomass and water use efficiency compared to other graft clones (i.e., male shoot scion and female rootstock, M/F; male shoot scion and male rootstock, M/M). This result suggests that grafting a female scion onto a male rootstock in P. cathayana was an effective method to mitigate water deficit stress to plants. Even so, the hypothesis needs to be tested in other poplar species in field environments because of the high variability in environmental stress tolerance in poplar species (Sixto et al. 2005). In a previous study, it was found that male P. deltoides are more abiotic-stress tolerant than females, while females grow better than males under non-stress conditions (Xu et al. 2016; Li et al. 2016). Based on these results, we hypothesize that graft clones (female scion and male rootstock) will have better growth and stronger tolerance to water deficit because grafting makes it possible to combine the desired rooting property traits of males with the desired above-ground traits of females. Thus far, little is known about the responses of reciprocal graft clones of P. deltoides to water deficit.
In this study, we investigated the effects of water deficit on plant growth and functional traits, soil microbial communities, and soil enzymatic activities of the reciprocal graft clones of P. deltoides. We hypothesized that there is a large set of parallel changes in above- and belowground responses when plants were exposed to water deficit, which could enhance the capability of plants to survive and grow during water deficits. Specifically, we tested the hypothesis that male-rooted graft clones will have stronger tolerance to water deficit, which is associated with an increase in root growth and relative abundance of drought-tolerant microbial groups such as gram-positive bacteria and fungi.
2 Materials and methods
2.1 Experimental design and plant materials
This study was conducted on a forest farm, located ~15 km north-west of Chengdu (30°39′12.5 N, 104°00′40.84E), southwest China. The region has a typical continental monsoon climate of the northern temperate zone with four distinct seasons, and elevation is approximately 520 m above sea level. The mean temperature ranges from 5 °C in winter to 26 °C in summer. The mean annual precipitation is 718 mm. Annual precipitation is concentrated mainly from April to June. The least amount of rainfall occurs mostly from December to March. The soil was classified as a mountain brown soil series (Chinese taxonomy). The soil properties were as follows: pH, 5.0; total N, 5.8 g kg−1; soil organic C, 85 g kg−1; and bulk density, 0.92 g cm−3.
We collected healthy male and female annual shoots of P. deltoides from the forest farm in the Jingkou District of Zhenjiang, Jiangsu Province, China (32°20′N, 119°47′E). Our present study focused on two genotypes of P. deltoides (one male and one female). In this approach, the degree of genetic divergence was low. The cuttings were cultivated in a hotbed in March 2016. One year later (March 2017), healthy, uniform approximately identical crown size and equal height (~15 cm) cuttings were selected for grafting. We employed a skilled technician to conduct the grafting. An experiment with four graft clones was planted in this area, consisting of two self-grafted P. deltoides clones, that is, self-grafted P. deltoides female shoot scion and female rootstock (F/F), self-grafted P. deltoides male shoot scion and male rootstock (M/M), and two reciprocally grafted P. deltoides clones [(i.e., female shoot scion and male rootstock (F/M), and male shoot scion and female rootstock (M/F)]. The experiment consisted of a factorial design of four graft clones and two water levels, with three replicates. A total 24 plots (plot area 0.4 m2, four plants per plot) were set up [two (water levels) × four (graft combinations) × three (replicates)]. Thus, it is three replicates with 4 seedlings per treatment combination per replicate, for a total of 12 seedlings per graft combination per treatment and 96 seedlings in total. Two watering regimes, irrigation and non-irrigation conditions (natural levels of precipitation) were supplied to every graft combination. In the irrigation treatment, the seedlings were irrigated with tap water to keep the soil volumetric water content at 35–45% (volumetric), which was an approximation of the optimal water content (Fig. 1). During the growing season, soil water content was measured weekly with a time-domain reflectometer (FieldScout TDR 100, Spectrum Technologies Inc., Aurora, IL, USA) at two locations around the seedlings over a depth of 0–20 cm. For each graft combination and treatment, six seedlings from three plots (two seedlings from each plot) were randomly selected for soil water measurements. The soil water content was calculated with means from individual replicates that subsampled two seedlings, resulting in n = 3 plots for each graft combination and treatment. Fertilizer was applied at a rate of 25 g N m−2, released evenly over the experimental period. Seedlings in the plots were arranged in a regular chessboard pattern of uniform composition, approximately equally spaced.
2.2 Determination of growth and plant photosynthetic capacity
For each graft combination and treatment, five plants from three blocks were selected for the growth and physiological parameter analysis. Plant height and stem diameter at collar were measured at the end of the experiment. Height was measured from the stem base to the top of the plant. Plants were harvested at the end of the experiment and divided into leaves, stems and roots. Biomass samples were dried (70 °C, 48 h) to constant weight and weighed. Root/shoot ratio was calculated using root and shoot dry weights. Total root length was measured using a scanner (V700, Epson, Long Beach, CA) and the image analysis system (WinRhizo, Regent Instruments, Quebec, Canada). The fine roots (defined as those less than 2 mm in diameter) were washed and oven-dried at 70 °C, weighed, and specific root length (SRL) was calculated as the ratio between the length of the fine roots and its dry weight. Samples (0.5 g) of fine roots were used to assay fine root activity using the triphenyltetrazolium chloride method, as described by Basile et al. (2007). The photosynthetic gas exchange measurements were carried out with a gas exchange system LI-6400 (Li-COR, Lincoln, NE, USA) between 08:00 and 11:30 during the experimental period. Net photosynthetic rates (Anet), and stomatal conductance (gs) were measured under the following conditions: air flux: 500 μmol s−1; temperature: 20–25 °C; vapor pressure deficit (VPD): 1.5 ± 0.2 kPa; photosynthetic photon flux density (PPFD):1500 μmol m−2 s−1; and ambient CO2 concentration, 400 μmol mol−1. Dried leaf samples were ground into a fine powder and passed through a mesh with a pore diameter of 250 μm. Leaf N concentrations were determined via flash combustion using a Carlo-Erba EA 1108 analyzer. Measurements of photosynthetic gas exchange parameters and leaf N were performed on 10 August 2017.
2.3 Soil sampling and analysis
Soil samples were collected from the topsoil (0–15 cm) in mid-September of 2017, representing the dry-heat season. For each graft combination and water treatment, four soil samples in each plot were randomly collected at 0–15 cm depth around the tree trunk by using soil-corer with an inner diameter of 2.5 cm and then combined into one composite sample. Each composite sample was sieved to a 2 mm mesh size to remove visible living plant material and stones. The samples were immediately transported to the laboratory in cooling boxes. Each collected soil sample was separately analyzed for phospholipid fatty acids after storage at −20 °C, and for physicochemical and enzyme assays samples were refrigerated at 4 °C. The remaining soil was air-dried, ground, and sieved (< 180 mm) to analyze the physical and chemical properties. The soil pH was determined in 1:5 (v/v) soil/ water mixed extracts with a pH electrode (FE20, Mettler Toledo, Greifensee, Switzerland). Soil extractable C was measured by the method described in Huang et al. (2008). The mixture in the tubes was then centrifuged at 3500 rpm for 15 min and filtered through a 0.45-μm filter membrane. Soil extractable C was measured with a TOC analyzer (Multi-N/C2100, Analytik Jena AG, Germany). Soil inorganic N ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N) were extracted with 2 M KCl and determined via a colorimetric method using a continuous flow-injection analyzer (SEAL AA3, Norderstedt, Germany). The nitrogen isotopic ratio (i.e., δ15N values) was defined as: [(atom%15Nsample—atom%15Nstandard)/atom%15Nstandard] × 1000. The δ15N of soil samples were determined on an isotope ratio mass spectrometer with a coupled system consisting of an elemental analyzer (Finnigan MAT-253, Thermo Electron). For 15N atmospheric nitrogen served as the primary standard. The standard deviation was <0.2 ‰ for δ15N.
The activities of extracellular enzymes β-1,4-N-acetyl-glucosaminidase (NAG) and leucine aminopeptidase (LAP) were assayed using fluorimetric assays. The activity of extracellular enzymes was measured using methylumbelliferone as the substrate with 365-nm excitation and 450-nm emission filters (Saiya-Cork et al. 2002). After correction for controls and quenching, enzyme activities were expressed as nmol g soil−1 h−1.
Phospholipid fatty acids (PLFAs) was used to characterize the soil microbial community using a modified method from Frostegård et al. (1991). Fresh soil samples (5 g) were extracted using chloroform: methanol: citrate buffer mixture (1:2:0.8, v/v/v) in three composite soil samples per grafting treatment. Phospholipids were transformed by alkaline methanolysis into fatty acid methyl esters (FAMEs) (Schindlbacher et al. 2011). The extraction with FAMEs was quantified using a Hewlett-Packard 6890 gas chromatography with capillary column Ultra 2-HP (cross-linked 5% phenyl-methyl silicone 25 m, 0.22 mm ID, 0.33 μm thickness), using hydrogen as a carrier gas. Fatty acids were quantified based on the 19:0 internal standard concentrations (Smithwick et al. 2005). The areas measured using a gas chromatography-flame ionization detector were used to calculate the abundance of individual fatty acids in nmol g−1 dry weight of the soil sample (Schindlbacher et al. 2011). The PLFAs i15:0, i16:0, i17:0, 18:1ω5c, 18:1ω7c, and 18:1ω9c were used as indicators for bacteria (Frostegård et al. 1993). We calculated the sum of a13:0, i13:0, a15:0, i15:0, a16:0, i16:0, a17:0, and i17:0 as markers for Gram-positive bacteria (O'Leary and Wilkinson 1988), whereas gram-negative bacteria were identified by the PLFAs: 2OH 16:1, 16:1ω7c, cy17:0, and cy19:0 (Federle 1986). The fungi were identified by the PLFAs 18:2ω6, 9c (Feng et al. 2010), and the PLFAs 10-Me16:0 and 10-Me18:0 were used as indicators for actinomycetes (Kanerva et al. 2008). The PLFAs 16:1ω5c, 16:1ω11 were used as representative of AMF (Royer-Tardif et al. 2010). As for the protozoa peak, 20:4ω6,9,12,15c and 20:5ω3,6,9,12,15 was used as the fatty acid signature (Mauclaire et al. 2003).
2.4 Statistical analysis
Statistical analyses were performed with SPSS 19.0 software (SPSS Inc., Chicago, IL). The data were checked for their normality, and homogeneity of variance and transformed when necessary. Differences in plant traits, soil enzyme activity, and soil microbial community structure were analyzed separately using linear mixed-effects models with fixed effects for grafting, water treatment, and their interactions and random effects for blocks. The effect of the block and the interactive effects of the block and other factors were not significant (P > 0.05) and therefore not presented. Tukey’s post-hoc test was used to examine the significant differences in the mean values between different treatments with a probability level of 0.05. Pearson’s correlation coefficients were used to test whether there were significant relationships among the measured microbial variables and soil properties.
3 Results
3.1 Soil properties and microbial enzyme activities
Soil moisture did not differ among graft clones but was significantly lower under water deficit (Fig. 1). The pH was significantly lower in M/M and F/M soil under water deficit. The water deficit significantly increased LAP and NAG activities in M/M and F/M soil (Table 1). The water deficit significantly decreased extractable C in F/F and M/F soil but tended to increase it in M/M and F/M soil. Consequently, extractable C, LAP and NAG activities in M/M and F/M soil were significantly greater than in F/F and M/F soil under water deficit (Table 1). The water deficit significantly decreased NH4+-N and NO3−-N pools in M/M and F/M soil (Table 1). The NH4+-N and NO3−-N pools were significantly lower in M/M and F/M soil than in F/F and M/F soil under water deficit (Table 1). Water deficits resulted in lower δ15N in M/M and F/M soil whereas there was no significant change in this parameter in F/F and M/F soil (Table 1).
3.2 Growth and biomass allocation
The four graft clones did not differ in height, diameter, total biomass, root biomass, root/shoot ratio, total root length, SRL and fine root activity under well-watered conditions (Fig. 2a–h). In contrast, water deficit decreased height, diameter, total biomass and fine root activity in the four graft clones, in particular, M/M and F/M (Fig. 2a–h). In M/M and F/M, water deficit increased root/shoot ratio and SRL. However, the water deficit did not affect these parameters in F/F and M/F (Fig. 2e, g). M/M and F/M had significantly greater height, diameter, total biomass, root biomass, root/shoot ratio, total root length, SRL, and fine root activities compared with F/F and M/F under water deficit (Fig. 2a–h).
Height, diameter, total biomass, root biomass, root/shoot ratio, total root length, specific root length (SRL) and fine root activity in four graft clones under well-watered and water deficit. Values of different letters represent significant differences (P < 0.05) in different graft clones. Error bars SE (n = 5 individuals)
3.3 Photosynthetic capacity
The four graft clones did not differ in Anet, gs and leaf N under well-watered conditions (Fig. 3a–c). Water deficit significantly decreased Anet, gs and leaf N in the four graft clones, in particular, in M/M and F/M. Compared with F/F and M/F, M/M and F/M had higher Anet, gs and leaf N under water deficit (Fig. 3a–c).
3.4 Soil microbial community composition
The content of bacteria, gram-negative, gram-positive and actinomycetes remained unaffected by the water deficit in the four graft clones (Fig. 4a, e–h). In contrast, water deficit significantly increased fungi PLFAs, AMF PLFAs and F/B in M/M and F/M soil. The M/M and F/M soil had a greater content of fungi PLFAs, AMF PLFAs, and F/B than in F/F and M/F soil under water deficit (Fig. 4b–d).
Soil microbial phospholipid fatty acids (PLFAs) at soil depths of 0–15 cm beneath four graft clones under well-watered and water deficit. Values of different letters represent significant differences (P < 0.05) in different graft clones. Error bars SE (n = 3 plots). F/B Ratio of fungal to bacterial PLFAs, G+ total gram-positive bacteria PLFAs, G− total gram-negative bacteria PLFAs
3.5 Correlations among potential PLFA signatures and environmental variables
Fungi PLFAs were positively correlated with EOC, NAG and LAP (r = 0.640, 0.608, and 0.671, respectively, all P < 0.01), and negatively correlated with NH4+-N, NO3−-N, soil δ15N, pH and soil moisture (r = −0.823, −0.670, −0.539, −0.682, and − 0.449, respectively, all P < 0.01). AMF PLFAs were positively correlated with soil extractable carbon (EOC), NAG and LAP (r = 0.633, 0.585, and 0.637, respectively, all P < 0.01) and negatively correlated with NH4+-N, NO3−-N, soil δ15 N, pH and soil moisture (r = −0.811, −0.709, −0.667, and − 0.673 and − 0.547, respectively, all P <0.01) across water regimes (Table 2).
4 Discussion
Poplars are among the fastest-growing trees, and their high productivity depends on water availability (Han et al. 2013). The concept of drought tolerance has been defined as the capacity to limit the decrease of biomass in response to drought (Passioura 2002, 2007). Accordingly, M/M and F/M are more tolerant to soil water deficit than F/F and M/F, as shown by the fact that the decreases in biomass and net photosynthetic rate were more accentuated in F/F and M/F in comparison with those in M/M and F/M. Large root biomass allocation and deep root systems have been identified as selection criteria for drought tolerance (Chapin et al. 1993; Smith et al. 2005). The root/shoot ratio in F/M and M/M increased under water deficit, largely due to a decrease in the growth of the shoots (data not shown) with little change in root biomass (Fig. 2d, e). Our results are in accord with other reports that a water deficit reduces shoot growth more than root growth (Lemoine et al. 2013; Xu et al. 2015). Despite little change in total root length, F/M and M/M responsive to soil water deficit are found to have greater SRL. Increased SRL might confer an advantage to optimize water and N acquisition by decreasing the plant carbon investment for root growth (Hernández et al. 2010; Comas et al. 2012).
Although the actinomycetal or protozoal biomass PLFAs were not significantly different in the soils among the four graft clones, the fungi biomarker 18:2ω6,9c and AMF biomarker 16:1ω5c and 16:1ω11 were strongly increased in F/M and M/M soil under water deficit. Similarly, it has been shown that different components of the microbial community showed different sensitivities to water deficit, with soil fungi being generally more tolerant of water stress than bacteria (Manzoni et al. 2012; Fuchslueger et al. 2014). Under water deficit, the increase of fungi PLFAs content in F/M and M/M soils coincides with higher soil extractable C, which is an important nutrition source for microbial growth (Huang et al. 2013). The higher soil extractable C in M/M and F/M soil can be associated directly with the higher specific root activity and SRL as observed in other studies (Darwent 2003; Yin et al. 2013). We suggest that F/M and M/M may increase soil extractable C compared to M/F and F/F, which may enhance microbial activity and stimulate fungi and mycorrhizal symbiosis in M/M and F/M. Consequently, increased fungal populations in M/M and F/M soil may improve plants access to nutrients and water through root networks (Smith et al. 2009; McGuire et al. 2010; Fuchslueger et al. 2014). This is reinforced by a smaller decrease in leaf N in M/M and F/M (Fig. 3c).
Natural 15N abundance, expressed as δ15N, relates to gross soil mineral N production rates (Högberg 1997; Huang et al. 2013). Generally, soil N mineralization, ammonia volatilization, nitrification, and denitrification discriminate against 15N, which allows the loss of isotopically light N and leaves isotopically heavy N in the soil (Högberg 1997). Consequently, high soil δ15N reflects faster N mineralization and losses through leaching and gaseous emission while low soil δ15N indicates lower rates of N transformation and less N output, and thus a relatively higher capacity for N retention (Hobbie and Ouimette 2009; Huang et al. 2013). In our study, under water deficit, the lower value of the soil δ15N in M/M and F/M indicates an enhancement in soil N retention capacity. The results were in agreement with those obtained by De Vries and Shade (2013) and Huang et al. (2013). They observed that soil microbial communities dominated by fungi corresponded with increased N retention and soil quality. Furthermore, the observed higher activities of N-releasing enzymes NAG and LAP in M/M and F/M soil than those in F/F and M/F soil under water deficit confirm an increase in soil quality in M/M and F/M soil. In our study, increased NAG activity is consistent with increased fungal populations. These findings are consistent with previous studies (Miller et al. 1998; Steinauer et al. 2015). In addition, there were significantly lower NH4+ and NO3− pools in M/M and F/M than in F/F and M/F soil under water deficit. This may imply that available N was efficiently taken up by the M/M and F/M, while plant N uptake was reduced in F/F and M/F due to reduction in growth under water deficit.
In conclusion, although we did not observe a much greater water deficit tolerance in F/M than M/M in terms of photosynthesis and growth, we found that male-rooted graft clones showed a larger extent of responsive changes to water shortage than female-rooted graft clones under water deficit. It is possible that the better performance of M/M and F/M, when exposed to water deficit, is associated mainly with higher SRL, root/shoot ratio and high fine root activity, which induce greater access to water and nutrients. Other acclimation strategies include increases in rhizosphere fungal growth, in association with high N-released enzyme activities. Increased rhizosphere may reflect positive plant-soil feedback that promotes growth in male-rooted graft clones with less N limitation under water deficit. This underlines that soil microbial communities and plant-soil interactions should be considered to better understand the mechanisms that create different levels of tolerance in plants to soil water deficit.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim JH, Allard G, Running SW, Semerci A, Cobb N (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684
Basile B, Bryla DR, Salsman ML, Marsal J, Cirillo C, Johnson RS, DeJong TM (2007) Growth patterns and morphology of fine roots of size-controlling and invigorating peach rootstocks. Tree Physiol 27:231–241
Cantero-Navarro E, Romero-Aranda R, Fernández-Muñoz R, Martínez-Andújar C, Pérez-Alfocea F, Albacete A (2016) Improving agronomic water use efficiency in tomato by rootstock-mediated hormonal regulation of leaf biomass. Plant Sci 251:90–100
Chapin FS, Autumn K, Pugnaire F (1993) Evolution of suites of traits in response to environmental stress. Am Nat 142:S78–S92
Ciais P, Reichstein M, Viovy N, Granier A, Ogee J, Allard V, Aubinet M, Buchmann N, Bernhofer C, Carrara A, Chevallier F, De Noblet N, Friend AD, Friedlingstein P, Grunwald T, Heinesch B, Keronen P, Knohl A, Krinner G, Loustau D, Manca G, Matteucci G, Miglietta F, Ourcival JM, Papale D, Pilegaard K, Rambal S, Seufert G, Soussana JF, Sanz MJ, Schulze ED, Vesala T, Valentini R (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533
Colla G, Rouphael Y, Cardarelli M, Salerno A, Rea E (2010) The effectiveness of grafting to improve alkalinity tolerance in watermelon. Environ Exp Bot 68:283–291
Comas LH, Mueller KE, Taylor LL, Midford PE, Callahan HS, Beerling DJ (2012) Evolutionary patterns and biogeochemical significance of angiosperm root traits. Int J Plant Sci 173:584–595
Darwent MJ (2003) Biosensor reporting of root exudation from Hordeum vulgare in relation to shoot nitrate concentration. J Exp Bot 54:325–334
De Vries FT, Shade A (2013) Controls on soil microbial community stability under climate change. Front Microbiol 4:265
Erismann ND, Machado EC, Tucci MLS (2008) Photosynthetic limitation by CO2 diffusion in drought stressed orange leaves on three rootstocks. Photosynth Res 96:163–172
Federle TW (1986) Microbial distribution in the soil-new techniques. In: Megusar F, Gantar M (eds) Perspectives in microbial ecology. Slovene Society for Microbiology, Ljubljana, pp 493–498
Fender AC, Gansert D, Jungkunst HF, Fiedler S, Beyer F, Schützenmeister K, Thiele B, Valtanen K, Polle A, Leuschner C (2013) Root-induced tree species effects on the source/sink strength for greenhouse gases (CH4, N2O and CO2) of a temperate deciduous forest soil. Soil Biol Biochem 57:587–597
Feng XJ, Simpson AJ, Schilesinger WH, Simpson MJ (2010) Altered microbial community structure and organic matter composition under elevated CO2 and N fertilization in the Duke forest. Glob Chang Biol 16:2104–2116
Frostegård Å, Tunlidb A, Bååth E (1991) Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Methods 14:151–163
Frostegård Å., Tunlidb A, Bååth E (1993) Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Fuchslueger L, Bahn M, Fritz K, Hasibeder R, Richter A (2014) Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytol 201:916–927
Gao Y, Tian Y, Liang X, Gao L (2015) Effects of single-root-grafting, double-root-grafting and compost application on microbial properties of rhizosphere soils in Chinese protected cucumber (Cucumis sativus L.) production systems. Sci Hortic 186:190–200
Han QQ, Luo JX, Li ZJ, Korpelainen H, Li CY (2018) Improved drought resistance by intergeneric grafting in Salicaceae plants under water deficits. Environ Exp Bot 155:217–225
Han Y, Wang Y, Jiang H, Wang M, Korpelainen H, Li C (2013) Reciprocal grafting separates the roles of the root and shoot in sex-related drought responses in Populus cathayana males and females. Plant Cell Environ 36:356–364
Hawkes CV, Kivlin SN, Rocca JD, Huguet V, Thomsen MA, Suttle KB (2011) Fungal community responses to precipitation. Glob Chang Biol 17:1637–1645
Hernández EI, Vilagrosa A, Pausas JG, Bellot J (2010) Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecol 207:233–244
Hobbie E, Ouimette A (2009) Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95:355–371
Högberg P (1997) 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Huang Z, Wan X, He Z, Yu Z, Wang M, Hu Z, Yang Y (2013) Soil microbial biomass, community composition and soil nitrogen cycling in relation to tree species in subtropical China. Soil Biol Biochem 62:68–75
Huang Z, Xu Z, Chen C (2008) Effect of mulching on labile soil organic matter pools, microbial community functional diversity and nitrogen transformations in two hardwood plantations of subtropical Australia. Appl Soil Ecol 40:229–239
Kanerva T, Palojärvi A, Rämö K, Manninen S (2008) Changes in soil microbial community structure under elevated tropospheric O3 and CO2. Soil Biol Biochem 40:2502–2510
Karlowsky S, Augusti A, Ingrisch J, Hasibeder R, Lange M, Lavorel S, Bahn M, Gleixner G (2018) Land use in mountain grasslands alters drought response and recovery of carbon allocation and plant-microbial interactions. J Ecol 106:1230–1243
Lemoine R, La Camera S, Atanassova R, Dedaldechamp F, Allario T, Pourtau N, Bonnemain JL, Laloi M, Coutos-Thevenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parrilla J, Durand M (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 4:272
Li Y, Duan B, Chen J, Korpelainen H, Niinemets Ü, Li C (2016) Males exhibit competitive advantages over females of Populus deltoides under salinity stress. Tree Physiol 12:1573–1584
Louws FJ, Rivard CL, Kubota C (2010) Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci Hortic 127:127–146
Machado DFSP, Ribeiro RV, Silveira JAG, Magalhães Filho JR, Machado EC (2013) Rootstocks induce contrasting photosynthetic responses of orange plants to low night temperature without affecting the antioxidant metabolism. Theor Exp Plant Physiol 25:26–35
Manzoni S, Schimel J, Porporato A (2012) Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93:930–938
Mariotte P, Robroek BJM, Jassey VEJ, Buttler A (2015) Subordinate plants mitigate drought effects on soil ecosystem processes by stimulating fungi. Funct Ecol 29:1578–1586
Martínez-Ballesta MC, Alcaraz-López C, Muries B, Mota-Cadenas C, Carvajal M (2010) Physiological aspects of rootstock-scion interactions. Sci Hortic 127:112–118
Martinez-Rodriguez MM, Estan MT, Moyano E, Garcia-Abellan JO, Flores FB, Campos JF, Al-Azzawi MJ, Flowers TJ, Bolarin MC (2008) The effectiveness of grafting to improve salt tolerance in tomato when an ‘excluder’ genotype is used as scion. Environ Exp Bot 63:392–401
Mauclaire L, Pelz O, Thullner M, Abraham W, Zeyer J (2003) Assimilation of toluene carbon along a bacteria-protist food chain determined by 13C-enrichment of biomarker fatty acids. J Microbiol Methods 55:635–649
McGuire KL, Bent E, Borneman J, Majumder A, Allison SD, Treseder KK (2010) Functional diversity in resource use by fungi. Ecology 91:2324–2233
Miller SS, Driscoll BT, Gregerson RG, Gantt JS, Vance CP (1998) Alfalfa malate dehydrogenase MDH: molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J 15:173–184
O'Leary WM, Wilkinson SG (1988) Gram-positive bacteria. In: Ratledge C, Wilkinson SG (eds) Microbial lipids, vol 1. Academic, London, pp 117–202
Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. J Exp Bot 58:113–117
Passioura JB (2002) Environmental biology and crop improvement. Funct Plant Biol 29:53–546
Paterson E, Midwood AJ, Millard P (2009) Through the eye of the needle: a review of isotope approaches to quantify microbial processes mediating soil carbon balance. New Phytol 184:19–33
Phillips RP, Meier IC, Bernhardt ES, Grandy AS, Wickings K, Finzi AC (2012) Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol Lett 15:1042–1049
Rodríguez-Gamir J, Primo-Millo E, Forner JB, Forner-Giner MA (2010) Citrus rootstock responses to water stress. Sci Hortic 126:95–102
Rouphael Y, Cardarelli M, Rea E, Colla G (2008) Grafting of cucumber as a means to minimize copper toxicity. Environ Exp Bot 63:49–58
Royer-Tardif S, Bradley RL, Parsons WFJ (2010) Evidence that plant diversity and site productivity confer stability to forest floor microbial biomass. Soil Biol Biochem 42:813–821
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Scheffers BR, De Meester L, Bridge TCL, Hoffmann AA, Pandolfi JM, Corlett RT, Butchart SHM, Pearce-Kelly P, Kovacs KM, Dudgeon D, Pacifici M, Rondinini C, Foden WB, Martin TG, Mora C, Bickford D, Watson JEM (2016) The broad footprint of climate change from genes to biomes to people. Science 354:aaf7671
Schindlbacher A, Rodler A, Kuffner M, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S (2011) Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biol Biochem 43:1417–1425
Schwarz D, Rouphael Y, Colla G, Venema JH (2010) Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Sci Hortic 127:162–171
Sixto H, Grau JM, Alba N, Alia R (2005) Response to sodium chloride in different species and clones of genus Populus L. Forestry 78:93–104
Smith DM, Inman-Bamber NG, Thorburn PJ (2005) Growth and function of the sugarcane root system. Field Crop Res 92:169–183
Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol 182:347–358
Smithwick EAH, Turner MG, Metzger KL, Balser TC (2005) Variation in NH4+ mineralization and microbial communities with stand age in lodgepole pine (Pinus contorta) forests, Yellowstone National Park (USA). Soil Biol Biochem 37:1546–1559
Steinauer K, Tilman D, Wragg PD, Cesarz S, Cowles JM, Pritsch K, Reich PB, Weisser WW, Eisenhauer N (2015) Plant diversity effects on soil microbial functions and enzymes are stronger than warming in a grassland experiment. Ecology 96:99–112
Tian Y, Zhang X, Liu J, Chen Q, Gao L (2009) Microbial properties of rhizosphere soils as affected by rotation, grafting, and soil sterilization in intensive vegetable production systems. Sci Hortic 123:139–147
Xu W, Cui K, Xu A, Nie L, Huang J, Peng S (2015) Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant 37:1–11
Xu X, Peng GQ, Wu CC, Korpelainen H, Li CY (2008) Drought inhibits photosynthetic capacity more in females than in males of Populus cathayana. Tree Physiol 28:1751–1759
Xu Z, Chen L, Tang S, Zhuang L, Yang W, Tu L, Tan B, Zhang L (2016) Sex-specific responses to Pb stress in Populus deltoids : root architecture and Pb translocation. Trees 30:2019–2027
Yin H, Li Y, Xiao J, Xu Z, Cheng X, Liu Q (2013) Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Glob Chang Biol 19:2158–2167
Zhang S, Jiang H, Peng SM, Korpelainen H, Li CY (2011) Sex-related differences in morphological, physiological, and ultrastructural responses of Populus cathayana to chilling. J Exp Bot 62:675–686
Zhao HX, Li Y, Zhang XL, Korpelainen H, Li CY (2012) Sex-related and stage-dependent source-to-sink transition in Populus cathayana grown at elevated CO2 and elevated temperature. Tree Physiol 32:1325–1338
Zhou B, Yin Y, Xu Y, Han L, Ye X, Bai L (2010) Effect of grafting on rhizospheric soil microorganisms and leaf NR activities of eggplants. Acta Hortic Sin 37:53–58
Funding
The research was supported by the Sichuan Science and Technology Support Program (N0.2017JY0234, SDS-135-1707).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Erwin Dreyer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contribution of the co-authors
Weilong Liu led the manuscript preparation with substantial contributions from all the coauthors. Yanping Wang, Xu Gong and Shuxin Li contributed substantially to the data collection. Joseph M. Smoak contributed significantly to the critical revisions of the manuscript. Baoli Duan supervised the research work and revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Liu, W., Wang, Y., Gong, X. et al. Changes in growth and soil microbial communities in reciprocal grafting clones between Populus deltoides males and females exposed to water deficit conditions. Annals of Forest Science 76, 118 (2019). https://doi.org/10.1007/s13595-019-0897-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-019-0897-9