Abstract
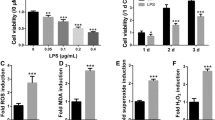
Periodontitis is an inflammatory disorder which leads to the defect of tooth-supporting tissue, especially in alveolar bone. During this process, the polarization behavior of macrophages affects immune inflammation and bone regeneration in which reactive oxygen species (ROS) play an essential role. ROS level should be regulated to the physiological level to protect stem cells from the inflammatory immune microenvironment. Our previous study constructed a ROS-responsive nanoplatform (Pssl-NAC), which possessed ROS-responsive antioxidative effect and could be potentially applied in periodontitis. However, the connection among bone regeneration, inflammation and oxidative stress remained in osteoimmune regulation is not clear. To further investigate the mechanism of the way how Pssl-NAC works in the treatment of periodontitis would be meaningful. Here, we investigated the effect of PssL-NAC in the regulation of the osteoimmune microenvironment through macrophage polarization. Results show PssL-NAC regulated the macrophage polarization direction in an inflammatory environment by maintaining an appropriate level of intracellular ROS, in which the MAPK/NFκB phosphorylation pathway is particularly important. In the macrophage-human periodontal ligament stem cells (hPDLSCs) co-culture system, PssL-NAC treatment significantly enhanced the osteogenic differentiation of hPDLSCs. In vivo experiment further confirmed the M2-like macrophages increased in the periodontal tissue of rats, and the expression of iNOS and p65 decreased after PssL-NAC treatment. In conclusion, PssL-NAC regulates the osteoimmune microenvironment and protects stem cells from oxidative stress injury for bone regeneration, which provides a strategy for the treatment of periodontitis.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44.
Weng Y, Wang H, Li L, Feng Y, Xu S, Wang Z. Trem2 mediated Syk-dependent ROS amplification is essential for osteoclastogenesis in periodontitis microenvironment. Redox Biol. 2021;40: 101849.
Sczepanik FSC, Grossi ML, Casati M, Goldberg M, Glogauer M, Fine N, Tenenbaum HC. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol. 2020;84(1):45–68.
Liu C, Mo L, Niu Y, Li X, Zhou X, Xu X. The role of reactive oxygen species and autophagy in periodontitis and their potential linkage. Front Physiol. 2017;8:439.
Herb M, Schramm M. Functions of ROS in macrophages and antimicrobial immunity. Antioxidants (Basel). 2021;10(2):313.
D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–24.
Qiu X, Yu Y, Liu H, Li X, Sun W, Wu W, Liu C, Miao L. Remodeling the periodontitis microenvironment for osteogenesis by using a reactive oxygen species-cleavable nanoplatform. Acta Biomater. 2021;135:593–605.
Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. 2019;19(10):626–42.
Yang D, Wan Y. Molecular determinants for the polarization of macrophage and osteoclast. Semin Immunopathol. 2019;41(5):551–63.
Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66.
Bashir S, Sharma Y, Elahi A, Khan F. Macrophage polarization: the link between inflammation and related diseases. Inflamm Res. 2016;65(1):1–11.
Bode JG, Ehlting C, Haussinger D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 2012;24(6):1185–94.
Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front Immunol. 2019;10:1084.
Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604.
Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016;2016:2795090.
Griess B, Mir S, Datta K, Teoh-Fitzgerald M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med. 2020;147:48–60.
Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23(7):898–914.
Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, Lu L, Yao Z, Goodman SB. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–9.
Criscitiello C, Viale G, Gelao L, Esposito A, De Laurentiis M, De Placido S, Santangelo M, Goldhirsch A, Curigliano G. Crosstalk between bone niche and immune system: osteoimmunology signaling as a potential target for cancer treatment. Cancer Treat Rev. 2015;41(2):61–8.
Shin RL, Lee CW, Shen OY, Xu H, Lee OK. The crosstalk between mesenchymal stem cells and macrophages in bone regeneration: a systematic review. Stem Cells Int. 2021;2021:8835156.
Huang RL, Yuan Y, Zou GM, Liu G, Tu J, Li Q. LPS-stimulated inflammatory environment inhibits BMP-2-induced osteoblastic differentiation through crosstalk between TLR4/MyD88/NF-kappaB and BMP/Smad signaling. Stem Cells Dev. 2014;23(3):277–89.
Sun X, Gao J, Meng X, Lu X, Zhang L, Chen R. Polarized macrophages in periodontitis: characteristics, function, and molecular signaling. Front Immunol. 2021;12: 763334.
Muri J, Kopf M. Redox regulation of immunometabolism. Nat Rev Immunol. 2021;21(6):363–81.
Tenorio M, Graciliano NG, Moura FA, Oliveira ACM, Goulart MOF. N-acetylcysteine (NAC): impacts on human health. Antioxidants (Basel). 2021;10(6):967.
Raghu G, Berk M, Campochiaro PA, Jaeschke H, Marenzi G, Richeldi L, Wen FQ, Nicoletti F, Calverley PMA. The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr Neuropharmacol. 2021;19(8):1202–24.
Xi X, Li ZX, Zhao Y, Liu H, Chen S, Liu DX. N-acetylcysteine promotes cyclic mechanical stress-induced osteogenic differentiation of periodontal ligament stem cells by down-regulating Nrf2 expression. J Dent Sci. 2022;17(2):750–62.
Zheng R, Tan Y, Gu M, Kang T, Zhang H, Guo L. N-acetyl cysteine inhibits lipopolysaccharide-mediated synthesis of interleukin-1beta and tumor necrosis factor-alpha in human periodontal ligament fibroblast cells through nuclear factor-kappa B signaling. Medicine (Baltimore). 2019;98(40):e17126.
Xiang P, Chen T, Mou Y, Wu H, Xie P, Lu G, Gong X, Hu Q, Zhang Y, Ji H. NZ suppresses TLR4/NF-kappaB signalings and NLRP3 inflammasome activation in LPS-induced RAW264.7 macrophages. Inflamm Res. 2015;64(10):799–808.
Li M, Dong L, Du H, Bao Z, Lin S. Potential mechanisms underlying the protective effects of Tricholoma matsutake singer peptides against LPS-induced inflammation in RAW264.7 macrophages. Food Chem. 2021;353:129452.
Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-Activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011: 792639.
Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48.
Huynh DTN, Jin Y, Van Nguyen D, Myung CS, Heo KS. Ginsenoside Rh1 inhibits angiotensin II-induced vascular smooth muscle cell migration and proliferation through suppression of the ROS-mediated ERK1/2/p90RSK/KLF4 signaling pathway. Antioxidants (Basel). 2022;11(4):643.
Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–85.
Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res. 2020;13:1057–73.
Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75(6):639–53.
Kumar SK, Mani KP. Endocan alters nitric oxide production in endothelial cells by targeting AKT/eNOS and NFkB/iNOS signaling. Nitric Oxide. 2021;117:26–33.
Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21(1):103–15.
Nakajima S, Kitamura M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radical Biol Med. 2013;65:162–74.
Choi JW, Kwon MJ, Kim IH, Kim YM, Lee MK, Nam TJ. Pyropia yezoensis glycoprotein promotes the M1 to M2 macrophage phenotypic switch via the STAT3 and STAT6 transcription factors. Int J Mol Med. 2016;38(2):666–74.
Ni C, Zhou J, Kong N, Bian T, Zhang Y, Huang X, Xiao Y, Yang W, Yan F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials. 2019;206:115–32.
Kang H, Lee MJ, Park SJ, Lee MS. Lipopolysaccharide-preconditioned periodontal ligament stem cells induce M1 polarization of macrophages through extracellular vesicles. Int J Mol Sci. 2018;19(12):3843.
Kats A, Gerasimcik N, Nareoja T, Nederberg J, Grenlov S, Lagnohed E, Desai S, Andersson G, Yucel-Lindberg T. Aminothiazoles inhibit osteoclastogenesis and PGE(2) production in LPS-stimulated co-cultures of periodontal ligament and RAW 264.7 cells, and RANKL-mediated osteoclastogenesis and bone resorption in PBMCs. J Cell Mol Med. 2019;23(2):1152–63.
Nagata M, Iwasaki K, Akazawa K, Komaki M, Yokoyama N, Izumi Y, Morita I. Conditioned medium from periodontal ligament stem cells enhances periodontal regeneration. Tissue Eng Part A. 2017;23(9–10):367–77.
Rajan TS, Giacoppo S, Trubiani O, Diomede F, Piattelli A, Bramanti P, Mazzon E. Conditioned medium of periodontal ligament mesenchymal stem cells exert anti-inflammatory effects in lipopolysaccharide-activated mouse motoneurons. Exp Cell Res. 2016;349(1):152–61.
Wu C, Chen Z, Wu Q, Yi D, Friis T, Zheng X, Chang J, Jiang X, Xiao Y. Clinoenstatite coatings have high bonding strength, bioactive ion release, and osteoimmunomodulatory effects that enhance in vivo osseointegration. Biomaterials. 2015;71:35–47.
Chen Z, Bachhuka A, Han S, Wei F, Lu S, Visalakshan RM, Vasilev K, Xiao Y. Tuning chemistry and topography of nanoengineered surfaces to manipulate immune response for bone regeneration applications. ACS Nano. 2017;11(5):4494–506.
Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19(6):1801.
Wang LX, Zhang SX, Wu HJ, Rong XL, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106(2):345–58.
Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223(4–5):383–96.
Kim HJ, Kim WJ, Ryoo HM. Post-translational regulations of transcriptional activity of RUNX2. Mol Cells. 2020;43(2):160–7.
Fierro FA, Nolta JA, Adamopoulos IE. Concise review: stem cells in osteoimmunology. Stem Cells. 2017;35(6):1461–7.
Okamoto K, Takayanagi H. Osteoimmunology. Cold Spring Harb Perspect Med. 2019;9(1):868.
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangsu Province under Grant [number BK20221177, BK20200665]; the “2015” Cultivation Program for Reserve Talents for Academic Leaders of Nanjing Stomatological School, Medical School of Nanjing University [number 0223A203, 0223A210]; Nanjing Clinical Research Center for Oral Diseases [number 2019060009]; and Key Project supported by Medical Science and Technology Development Foundation, Nanjing Department of Health under Grant [number ZKX22055, YKK20153].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the standards upheld by the Ethics Committee of Nanjing Stomatological Hospital, Affiliated Hospital of Medical School. All animal experiments were approved by the Ethics Committee of Nanjing Stomatological Hospital, Affiliated Hospital of Medical School for the use of animals and conducted in accordance with the National Institutes of Health Laboratory Animal Care and Use Guidelines (Approval No. NJSH-2022NL-009).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, X., Peng, H., Zhao, Y. et al. Remodeling periodontal osteoimmune microenvironment through MAPK/NFκB phosphorylation pathway of macrophage via intelligent ROS scavenging. Human Cell 36, 1991–2005 (2023). https://doi.org/10.1007/s13577-023-00979-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00979-3