Abstract
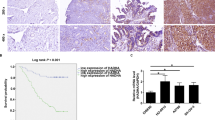
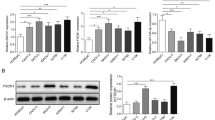
Ovarian cancer is the second most common cause of gynecological cancer death and has a high recurrence rate. FOXN3, a transcription inhibitor belonging to FOX family, has anti-tumor effects on several cancers. Bioinformatics analysis revealed that the expression of FOXN3 was downregulated in ovarian cancer specimens. However, the role of FOXN3 in ovarian cancer remains unclear. Herein, we investigated the role of FOXN3 in ovarian cancer using OVCAR3 and A2780 cells. Flow cytometry and CCK-8 analysis showed that overexpression of FOXN3 inhibited the proliferation and cell cycle progression of OVCAR3 cells. Cell invasion and migration abilities were decreased by FOXN3 according to transwell and wound healing assays. The suppression of FOXN3 on angiogenesis in OVCAR3 cells was evidenced by reduced vessel formation and VEGFA protein expression. Taken together, FOXN3 had an inhibitory effect on the proliferation, migration, invasion and angiogenesis of OVCAR3 cells, while its knockdown exhibited an opposite effect in A2780 cells. By inoculation of FOXN3-overexpressing cells into nude mice, tumorigenesis assay demonstrated that FOXN3 could delay the growth of ovarian cancer cells in vivo. The interaction between FOXN3 and RPS15A was preliminarily explored via dual-luciferases assay and ChIP. FOXN3 was confirmed to bind to the promoter (at − 1588/− 1581 and − 1476/− 1467) of gene RPS15A and inhibit its transcriptional expression. We further found that overexpression of RPS15A diminished the inhibition of FOXN3 on ovarian cancer cell malignant behaviors. These findings indicate that FOXN3 negatively regulates the expression of RPS15A and thus suppresses the progression of ovarian cancer.








Similar content being viewed by others
Data availability
All data generated during the current study are included in the article.
References
Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009;80(6):609–16.
Penny SM. Ovarian cancer: an overview. Radiol Technol. 2020;91(6):561–75.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–96. https://doi.org/10.3322/caac.21456.
Dubey JP, Lindsay DS, Speer CA, Fayer R, Livingston CW Jr. Sarcocystis arieticanis and other Sarcocystis species in sheep in the United States. J Parasitol. 1988;74(6):1033–8.
Liu C, He X, Liu X, Yu J, Zhang M, Yu F, et al. RPS15A promotes gastric cancer progression via activation of the Akt/IKK-beta/NF-kappaB signalling pathway. J Cell Mol Med. 2019;23(3):2207–18. https://doi.org/10.1111/jcmm.14141.
Kong L, Wei Q, Hu X, Chen L, Li J. Ribosomal protein small subunit 15A (RPS15A) inhibits the apoptosis of breast cancer MDA-MB-231 cells via upregulating phosphorylated ERK1/2, Bad, and Chk1. J Cell Biochem. 2020;121(1):587–95. https://doi.org/10.1002/jcb.29304.
Zhao X, Shen L, Feng Y, Yu H, Wu X, Chang J, et al. Decreased expression of RPS15A suppresses proliferation of lung cancer cells. Tumour Biol. 2015;36(9):6733–40. https://doi.org/10.1007/s13277-015-3371-9.
Guo P, Wang Y, Dai C, Tao C, Wu F, Xie X, et al. Ribosomal protein S15a promotes tumor angiogenesis via enhancing Wnt/beta-catenin-induced FGF18 expression in hepatocellular carcinoma. Oncogene. 2018;37(9):1220–36. https://doi.org/10.1038/s41388-017-0017-y.
Yang H, Qi Y, Wang XL, Gu JJ, Shi TM. Down-regulation of lncRNA BLACAT1 inhibits ovarian cancer progression by suppressing the Wnt/beta-catenin signaling pathway via regulating miR-519d-3p. Mol Cell Biochem. 2020;467(1–2):95–105. https://doi.org/10.1007/s11010-020-03704-y.
Kong X, Zhai J, Yan C, Song Y, Wang J, Bai X, et al. Recent advances in understanding FOXN3 in breast cancer, and other malignancies. Front Oncol. 2019;9:234. https://doi.org/10.3389/fonc.2019.00234.
Li W, Zhang Z, Liu X, Cheng X, Zhang Y, Han X, et al. The FOXN3-NEAT1-SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J Clin Invest. 2017;127(9):3421–40. https://doi.org/10.1172/JCI94233.
Peng Q, Zhang L, Li J, Wang W, Cai J, Ban Y, et al. FOXA1 Suppresses the Growth, migration, and invasion of nasopharyngeal carcinoma cells through repressing miR-100-5p and miR-125b-5p. J Cancer. 2020;11(9):2485–95. https://doi.org/10.7150/jca.40709.
Xu Z, Yang Y, Li B, Li Y, Xia K, Yang Y, et al. Checkpoint suppressor 1 suppresses transcriptional activity of ERalpha and breast cancer cell proliferation via deacetylase SIRT1. Cell Death Dis. 2018;9(5):559. https://doi.org/10.1038/s41419-018-0629-3.
Sun J, Li H, Huo Q, Cui M, Ge C, Zhao F, et al. The transcription factor FOXN3 inhibits cell proliferation by downregulating E2F5 expression in hepatocellular carcinoma cells. Oncotarget. 2016;7(28):43534–45. https://doi.org/10.18632/oncotarget.9780.
Hamilton TC, Young RC, McKoy WM, Grotzinger KR, Green JA, Chu EW, et al. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983;43(11):5379–89.
Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280–304. https://doi.org/10.3322/caac.21559.
Xue W, Ma L, Wang Z, Zhang W, Zhang X. FOXN3 is downregulated in osteosarcoma and transcriptionally regulates SIRT6, and suppresses migration and invasion in osteosarcoma. Oncol Rep. 2019;41(2):1404–14. https://doi.org/10.3892/or.2018.6878.
Li Q, Li X, Guo Z, Xu F, Xia J, Liu Z et al. MicroRNA-574–5p was pivotal for TLR9 signaling enhanced tumor progression via down-regulating checkpoint suppressor 1 in human lung cancer. PLoS One. 2012;7(11):e48278. doi:https://doi.org/10.1371/journal.pone.0048278.
Markowski J, Tyszkiewicz T, Jarzab M, Oczko-Wojciechowska M, Gierek T, Witkowska M, et al. Metal-proteinase ADAM12, kinesin 14 and checkpoint suppressor 1 as new molecular markers of laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2009;266(10):1501–7. https://doi.org/10.1007/s00405-009-1019-3.
Ying H, Lyu J, Ying T, Li J, Jin S, Shao J, et al. Risk miRNA screening of ovarian cancer based on miRNA functional synergistic network. J Ovarian Res. 2014;7:9. https://doi.org/10.1186/1757-2215-7-9.
Dai Y, Wang M, Wu H, Xiao M, Liu H, Zhang D. Loss of FOXN3 in colon cancer activates beta-catenin/TCF signaling and promotes the growth and migration of cancer cells. Oncotarget. 2017;8(6):9783–93. https://doi.org/10.18632/oncotarget.14189.
Scott KL, Plon SE. CHES1/FOXN3 interacts with Ski-interacting protein and acts as a transcriptional repressor. Gene. 2005;359:119–26. https://doi.org/10.1016/j.gene.2005.06.014.
Katayama M, Hirai S, Kamihagi K, Nakagawa K, Yasumoto M, Kato I. Soluble E-cadherin fragments increased in circulation of cancer patients. Br J Cancer. 1994;69(3):580–5. https://doi.org/10.1038/bjc.1994.106.
Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. https://doi.org/10.1016/j.critrevonc.2017.11.010.
Zhou P, Xiong T, Chen J, Li F, Qi T, Yuan J. Clinical significance of melanoma cell adhesion molecule CD146 and VEGFA expression in epithelial ovarian cancer. Oncol Lett. 2019;17(2):2418–24. https://doi.org/10.3892/ol.2018.9840.
Premalata CS, Umadevi K, Shobha K, Anurekha M, Krishnamoorthy L. Expression of VEGF-A in epithelial ovarian cancer: correlation with morphologic types, grade and clinical stage. Gulf J Oncolog. 2016;1(21):49–54.
Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):114–27. https://doi.org/10.1111/joim.12019.
Wan BS, Wang XY, Tiang J, Zhou C, Lin J, Wang Z. Ribosomal protein RPS15A augments proliferation of colorectal cancer RKO cells via regulation of BIRC3, p38 MAPK and Chk1. Eur Rev Med Pharmacol Sci. 2021;25(11):3967–80. https://doi.org/10.26355/eurrev_202106_26037.
Liang J, Liu Z, Zou Z, Wang X, Tang Y, Zhou C, et al. Knockdown of ribosomal protein S15A inhibits human kidney cancer cell growth in vitro and in vivo. Mol Med Rep. 2019;19(2):1117–27. https://doi.org/10.3892/mmr.2018.9751.
Xu W, Li Y, Ye X, Ji Y, Chen Y, Zhang X, et al. TMED3/RPS15A Axis promotes the development and progression of osteosarcoma. Cancer Cell Int. 2021;21(1):630. https://doi.org/10.1186/s12935-021-02340-w.
Huot G, Vernier M, Bourdeau V, Doucet L, Saint-Germain E, Gaumont-Leclerc MF, et al. CHES1/FOXN3 regulates cell proliferation by repressing PIM2 and protein biosynthesis. Mol Biol Cell. 2014;25(5):554–65. https://doi.org/10.1091/mbc.E13-02-0110.
Mudduluru G, Abba M, Batliner J, Patil N, Scharp M, Lunavat TR, et al. A Systematic approach to defining the microRNA landscape in metastasis. Cancer Res. 2015;75(15):3010–9. https://doi.org/10.1158/0008-5472.CAN-15-0997.
Karanth S, Zinkhan EK, Hill JT, Yost HJ, Schlegel A. FOXN3 regulates hepatic glucose utilization. Cell Rep. 2016;15(12):2745–55. https://doi.org/10.1016/j.celrep.2016.05.056.
Kurman RJ, Shih IM. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–47. https://doi.org/10.1016/j.ajpath.2015.11.011.
Hollis RL. Molecular characteristics and clinical behaviour of epithelial ovarian cancers. Cancer Lett. 2023;555:216057. doi:https://doi.org/10.1016/j.canlet.2023.216057.
Wang C, Tu H, Yang L, Ma C, Hu J, Luo J, et al. FOXN3 inhibits cell proliferation and invasion via modulating the AKT/MDM2/p53 axis in human glioma. Aging (Albany NY). 2021;13(17):21587–98. https://doi.org/10.18632/aging.203499.
Chen J, Wei Y, Feng Q, Ren L, He G, Chang W, et al. Ribosomal protein S15A promotes malignant transformation and predicts poor outcome in colorectal cancer through misregulation of p53 signaling pathway. Int J Oncol. 2016;48(4):1628–38. https://doi.org/10.3892/ijo.2016.3366.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant No. 82001846) and the 345 Talent Project of Shengjing Hospital of China Medical University.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 82001846) and the 345 Talent Project of Shengjing Hospital of China Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
All clinical studies and animal studies were approved by the ethics committee of Shengjing Hospital of China Medical University. All animal experiments were performed by the Guide for the Care and Use of Laboratory Animals. The clinical studies in human were conducted in accordance with the principles of the Helsinki Declaration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Li, M. & Qi, Y. FOXN3 inhibits the progression of ovarian cancer through negatively regulating the expression of RPS15A. Human Cell 36, 1120–1134 (2023). https://doi.org/10.1007/s13577-023-00876-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00876-9